Difference between revisions of "Cold climate impact on runoff management"
| Line 29: | Line 29: | ||
<p>The complex melting pattern that occurs within a snowpack results in the release of pollutants at different times during the melt, further complicating an already difficult management scenario. The variability of snow character and the repeated freeze-thaw cycles that occur throughout a long winter create a very heterogeneous snowpack, with many different flow paths available for melt water to move along. The freeze-thaw cycles also result in the re-crystallization of snow and the subsequent exclusion of “impurities” to the outside edge of the crystals, whereupon they become available for wash-off by the melting front as it passes. The process has been called by many different names, including “preferential elution,” “freeze extraction,” and “first flush.” This melting sequence becomes a very important part of snowmelt quality management because the practices used may or may not come close to treating a particular target pollutant depending upon where in the sequence it is captured.</p> | <p>The complex melting pattern that occurs within a snowpack results in the release of pollutants at different times during the melt, further complicating an already difficult management scenario. The variability of snow character and the repeated freeze-thaw cycles that occur throughout a long winter create a very heterogeneous snowpack, with many different flow paths available for melt water to move along. The freeze-thaw cycles also result in the re-crystallization of snow and the subsequent exclusion of “impurities” to the outside edge of the crystals, whereupon they become available for wash-off by the melting front as it passes. The process has been called by many different names, including “preferential elution,” “freeze extraction,” and “first flush.” This melting sequence becomes a very important part of snowmelt quality management because the practices used may or may not come close to treating a particular target pollutant depending upon where in the sequence it is captured.</p> | ||
<p>Melt water can move downward in a snowpack through different flow-paths around “dry” snow and ice layers caused by repeated freeze-thaw cycles. As this water moves through, it picks up or mobilizes soluble ions that have pushed to the edge of ice crystals. Through this process, the snowpack cleanses itself of soluble contaminants that become available in the first phases of the melt, yielding a highly soluble, acidic and perhaps toxic (to animal and plant-life) runoff volume. Later in the event, melt water from the snowpack is depleted in these soluble contaminants, but water flow can be at its highest. Energy levels are only high enough to move fine- to medium-grained particulates when the snowpack allows their passage, leaving behind the coarser-grained material. The coarser material is available for wash-off during the higher energy spring rainfall events, and becomes a major source of contamination at that time. Alert sweeping can pick this coarse material up from paved surfaces if there is an opportunity between the departure of snow and the first rains.</p> | <p>Melt water can move downward in a snowpack through different flow-paths around “dry” snow and ice layers caused by repeated freeze-thaw cycles. As this water moves through, it picks up or mobilizes soluble ions that have pushed to the edge of ice crystals. Through this process, the snowpack cleanses itself of soluble contaminants that become available in the first phases of the melt, yielding a highly soluble, acidic and perhaps toxic (to animal and plant-life) runoff volume. Later in the event, melt water from the snowpack is depleted in these soluble contaminants, but water flow can be at its highest. Energy levels are only high enough to move fine- to medium-grained particulates when the snowpack allows their passage, leaving behind the coarser-grained material. The coarser material is available for wash-off during the higher energy spring rainfall events, and becomes a major source of contamination at that time. Alert sweeping can pick this coarse material up from paved surfaces if there is an opportunity between the departure of snow and the first rains.</p> | ||
| − | |||
| − | |||
[[File:Percolation of water through snowpack.jpg|thumb|300px|alt=Percolation of water through snowpack|<font size=3>Percolation of water through snowpack</font size>]] | [[File:Percolation of water through snowpack.jpg|thumb|300px|alt=Percolation of water through snowpack|<font size=3>Percolation of water through snowpack</font size>]] | ||
| + | <p>The management implication of the preferential elution (or chemical dissolution) process is illustrated in Figure 9.4. The graphic shows that the early part of the melt involves the very efficient elution of soluble constituents (Cl, dissolved metals and nutrients, dissolved organics, e.g.) at the crystal edges, resulting in a substantial release of the soluble component of a snowpack, often resulting in a “shock” effect as these pollutants reach a receiving water body. Following the release of solubles is a period when much of the liquid volume of the snowpack releases (skewed toward the earlier part of the mid-melt event) and carries with it the remaining solubles along with the beginning portion of finer-grained solids and associated contaminants (hydrophobic PAHs, e.g.). This mid-melt period generally has the largest portion of water runoff associated with the melt, and the mobilization of solids begins and continues as long as sufficient energy is available to move the finer particles, leaving behind the larger particles.</p> | ||
| + | <p>Part of the severity of the water quality problem associated with melt is that it occurs when the hydrologic system is least able to deal with it. Routine assumptions on biological activity, aeration, settling, and pollutant degradation are altered by the cold temperatures, cold water and ice covered conditions that prevail for many months. An end of the season rain-on-snow event often presents the worst-case scenario when rain falls onto a deep, possibly saturated snowpack. The movement of a well defined, rapidly moving wetted front through the snowpack results in the mobilization of soluble constituents, plus the energy associated with the rainfall is sufficient to mobilize the fine-grained or possibly larger solids and associated contaminants. This wave of melt also washes over urban surfaces and picks up material that has been deposited on these surfaces all winter. Comprehensive reviews of the quality of snowmelt are presented in many of the references at the end of this chapter.</p> | ||
<p>The toxicity of the meltwater and the effects that these chemicals have on various receiving waters and related biological resources is still poorly understood. It is understood that meltwater can be extremely concentrated in many different toxic substances (metals, PAHs, organics, free cyanide, chloride). However, little is known about the impact of these substances on streams, lakes, ground water and wetlands, and even less about their impact on plants, invertebrates, fish and other biological life.</p> | <p>The toxicity of the meltwater and the effects that these chemicals have on various receiving waters and related biological resources is still poorly understood. It is understood that meltwater can be extremely concentrated in many different toxic substances (metals, PAHs, organics, free cyanide, chloride). However, little is known about the impact of these substances on streams, lakes, ground water and wetlands, and even less about their impact on plants, invertebrates, fish and other biological life.</p> | ||
<p>The effects of road salting, especially the conservative element chloride (Cl), becomes increasingly important as the number of vehicles in the state dramatically increases. With the increased number of vehicles comes a need to provide ever safer traffic-ways, which translates into ice-free roads for several months in cold climates. The increase in road salt has even led the Government of Canada to recommend the inclusion of Cl as a toxic substance because of the impact of this chemical on ground and surface waters. Associated with Cl is the anti-caking salt additive, sodium ferrocyanide, which is used commonly in Minnesota. Although not toxic itself, ferrocyanide can break down to free cyanide, which is extremely toxic at low levels. Recent data collected in Minnesota has shown that chemicals associated with salting operations (Na, Cl and cyanide) can reach very high levels in runoff from sites where salt is stored and handled, even if recommended handling procedures are followed.</p> | <p>The effects of road salting, especially the conservative element chloride (Cl), becomes increasingly important as the number of vehicles in the state dramatically increases. With the increased number of vehicles comes a need to provide ever safer traffic-ways, which translates into ice-free roads for several months in cold climates. The increase in road salt has even led the Government of Canada to recommend the inclusion of Cl as a toxic substance because of the impact of this chemical on ground and surface waters. Associated with Cl is the anti-caking salt additive, sodium ferrocyanide, which is used commonly in Minnesota. Although not toxic itself, ferrocyanide can break down to free cyanide, which is extremely toxic at low levels. Recent data collected in Minnesota has shown that chemicals associated with salting operations (Na, Cl and cyanide) can reach very high levels in runoff from sites where salt is stored and handled, even if recommended handling procedures are followed.</p> | ||
Revision as of 15:11, 21 March 2013
This section introduces national and international research and experience on stormwater practices maintained in cold climate regions, and presents principles for adapting BMPs to provide effective pollutant removal and runoff control during cold-weather months. It also introduces some recent findings from within Minnesota on the impact of climate change on stormwater and meltwater runoff. For more information on this topic, see Chapter 5 of MPCA’s Protecting Water Quality in Urban Areas.
Minnesota stormwater managers must recognize that runoff from snowmelt has characteristics different than those of rainfall runoff, and that BMP design criteria addressing only rainfall runoff might not work well during cold periods. This becomes a major problem because a substantial percentage of annual runoff volume and loading can come from snowmelt in years when snowfall is high.
Contents
- 1 Nature of the cold climate problem
- 2 Challenges in engineering and design
- 3 Management approaches
- 4 Design adaptations for cold climates
- 5 Preliminary considerations for design sheets based on cold climate performance
- 5.1 Applicability of BMPs for Cold Climate
- 5.2 Adaptation concepts
- 5.3 Infiltration basin/surface filter
- 5.4 Seasonal ponds
- 5.5 The importance of baseflow, inlet and outlet design in ponds
- 5.6 Bioretention
- 5.7 Vegetated conveyance
- 5.8 Snow and ice management
- 5.9 High sediment load
- 5.10 Secondary practices
- 6 References
- 7 Annotated Bibliography
Nature of the cold climate problem
Hydrology of melt
The heart of the problem with snowmelt runoff is that water volume in the form of snow and ice builds for several months and suddenly releases with the advent of warm weather in the spring or during short interim periods all winter long. The interim melts generally do not contribute a significant volume of runoff when compared to the large spring melt. Note that snowmelt peaks are substantially less than those from rainfall, but the total event volume of a snowmelt, although it occurs over a much longer period, can be substantially more. Ignoring the contribution of these large, spring melts to the annual runoff and pollution loading analysis could be a major omission in a watershed analysis. This type of comparison also shows why facility design is critical to the proper quantity and quality management of this meltwater.
This behavior of seeing a major portion of the annual runoff occur during the relatively short period in the year when the snowpack melts is typical of cold climates. Factors influencing the nature of this melt and the speed with which it occurs include solar radiation, the distribution of snow cover, the addition of de-icing chemicals to the pack, and the amount of freeze-thaw cycling.
The source area for snowmelt plays a critical role in both the hydrologic and water quality character of snowmelt runoff. Roadways and large paved surfaces like commercial parking lots are the direct recipients of fast and efficient snow removal. This can occur by plowing, which can include total site removal or relocation off of the surface, and/or chemical-induced (salt) melting. Because of the need to promote safety, obtaining an ice- and snow-free surface is a focal point for winter management of these surfaces. As a result, these surfaces generate numerous loading events every time it snows or even in anticipation of a snowfall, since pre-icing application of salt can be a common practice. By the time the major spring melt occurs, many of these surfaces are free of snow and ice. However, in many instances the snow that has been removed is piled or plowed close to the surface and flows onto it. At this time it becomes part of the urban drainage system or is stored in a location where it immediately enters the drainage system upon melt. These road and parking surfaces can be a significant source of many of the most contaminating pollutants associated with urban runoff.
A second category of importance to snowmelt runoff is the area immediately adjacent to the roadway or parking surface. This is the area that is generally the most significant source of poor water quality during a melt. Because snow is plowed and piled in these areas, they accumulate both equivalent water volume and pollutants for an extended period of time over the winter. This material is then available for release and migration over a several week period in the spring. This critical area is usually contained within about 25 feet of the paved surface and easily flows to the storm drain system as it melts. Sometimes, as in commercial parking or roadside piles, the snow is actually sitting on an impervious surface.
The final contributing area to meltwater runoff is the less developed residential, open space, low density area typical of suburban watersheds. An example of these includes areas well removed from roadways and high traffic. Snowmelt from these areas can be large contributors of meltwater volume, but the quality of the melt is better than from roadways and parking areas. Typically a fair amount of the initial meltwater soaks into the ground and can continue to do so as long as the rate of melt does not exceed the infiltration capacity of the soil. If sufficient snowpack is available, saturation can occur, leading to this portion of the watershed acting as an impervious surface.
The relative contributions of the three principal areas shown in these photos cannot be generalized because of the mix that occurs within any watershed. However, the characteristics of a specific watershed and the management approach needed as a result can be estimated from the mix. That is, a densely developed urban area will have more roadways and impervious parking surfaces typical of paved surfaces with heavy traffic, whereas a suburban neighborhood or rural setting will be a larger source of volume.
Snowpack builds throughout the winter and increases in moisture content as the winter season draws toward melt. When meltwater exits the snowpack, it moves into the ground or over the land surface. A very important part of snowmelt management for both quantity and quality depends upon this behavior and the variations it takes. It is very common for the first part of the melt to soak into the ground. However, at some point in the melt sequence, particularly when there is a deep snowpack at the on-set, the ground can become saturated and turn a pervious, non-contributing part of the watershed into an essentially impervious surface from which all additional melt runs off. Hydrographs from melt events will typically show a period of little to no runoff, even though the melt rate might be high, followed by accelerating flow as the ground no longer soaks in the melting snowpack. Recognizing this behavior could be important for early runoff and quality management.
Quality of melt
The water quality problems associated with melt occur because the large volume of water released during melt and rain-on-snow events not only carries with it the material accumulated in the snowpack all winter, but also material it picks up as it flows over the land’s surface. The winter accumulation can occur directly on a standing snowpack or on the side of a roadway where it is plowed. In either case, the material builds for several months prior to wash-off. Since snow is a very effective scavenger of atmospheric pollutants, literally any airborne material present in a snow catchment will show up in meltwater when it runs off. Add to this the material applied to, or deposited upon the land surface, for example to melt snow or prevent cars from sliding, and the wide range of potential pollutants becomes apparent. As with the volume of meltwater, a major portion of annual pollutant loading can be associated with spring melt events.
The conventional pollutants of concern for most urban runoff situations are supplemented in meltwater runoff by additional contaminants added during the winter. The solids, nutrients, and metals present during the summer are joined by increased polynuclear aromatic hydrocarbons (PAHs) and hydrocarbons from inefficient and increased fuel combustion; by salt and increased solids from anti-skid application; and by cyanide that has been added as an anti-caking additive to salt. Pesticide and fertilizer runoff and organic debris (leaves, grass clippings, seeds) are less of a concern during the winter.
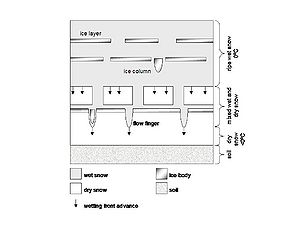
The complex melting pattern that occurs within a snowpack results in the release of pollutants at different times during the melt, further complicating an already difficult management scenario. The variability of snow character and the repeated freeze-thaw cycles that occur throughout a long winter create a very heterogeneous snowpack, with many different flow paths available for melt water to move along. The freeze-thaw cycles also result in the re-crystallization of snow and the subsequent exclusion of “impurities” to the outside edge of the crystals, whereupon they become available for wash-off by the melting front as it passes. The process has been called by many different names, including “preferential elution,” “freeze extraction,” and “first flush.” This melting sequence becomes a very important part of snowmelt quality management because the practices used may or may not come close to treating a particular target pollutant depending upon where in the sequence it is captured.
Melt water can move downward in a snowpack through different flow-paths around “dry” snow and ice layers caused by repeated freeze-thaw cycles. As this water moves through, it picks up or mobilizes soluble ions that have pushed to the edge of ice crystals. Through this process, the snowpack cleanses itself of soluble contaminants that become available in the first phases of the melt, yielding a highly soluble, acidic and perhaps toxic (to animal and plant-life) runoff volume. Later in the event, melt water from the snowpack is depleted in these soluble contaminants, but water flow can be at its highest. Energy levels are only high enough to move fine- to medium-grained particulates when the snowpack allows their passage, leaving behind the coarser-grained material. The coarser material is available for wash-off during the higher energy spring rainfall events, and becomes a major source of contamination at that time. Alert sweeping can pick this coarse material up from paved surfaces if there is an opportunity between the departure of snow and the first rains.
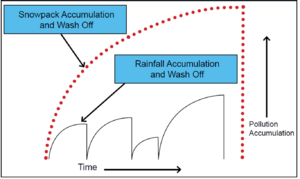
The management implication of the preferential elution (or chemical dissolution) process is illustrated in Figure 9.4. The graphic shows that the early part of the melt involves the very efficient elution of soluble constituents (Cl, dissolved metals and nutrients, dissolved organics, e.g.) at the crystal edges, resulting in a substantial release of the soluble component of a snowpack, often resulting in a “shock” effect as these pollutants reach a receiving water body. Following the release of solubles is a period when much of the liquid volume of the snowpack releases (skewed toward the earlier part of the mid-melt event) and carries with it the remaining solubles along with the beginning portion of finer-grained solids and associated contaminants (hydrophobic PAHs, e.g.). This mid-melt period generally has the largest portion of water runoff associated with the melt, and the mobilization of solids begins and continues as long as sufficient energy is available to move the finer particles, leaving behind the larger particles.
Part of the severity of the water quality problem associated with melt is that it occurs when the hydrologic system is least able to deal with it. Routine assumptions on biological activity, aeration, settling, and pollutant degradation are altered by the cold temperatures, cold water and ice covered conditions that prevail for many months. An end of the season rain-on-snow event often presents the worst-case scenario when rain falls onto a deep, possibly saturated snowpack. The movement of a well defined, rapidly moving wetted front through the snowpack results in the mobilization of soluble constituents, plus the energy associated with the rainfall is sufficient to mobilize the fine-grained or possibly larger solids and associated contaminants. This wave of melt also washes over urban surfaces and picks up material that has been deposited on these surfaces all winter. Comprehensive reviews of the quality of snowmelt are presented in many of the references at the end of this chapter.
The toxicity of the meltwater and the effects that these chemicals have on various receiving waters and related biological resources is still poorly understood. It is understood that meltwater can be extremely concentrated in many different toxic substances (metals, PAHs, organics, free cyanide, chloride). However, little is known about the impact of these substances on streams, lakes, ground water and wetlands, and even less about their impact on plants, invertebrates, fish and other biological life.
The effects of road salting, especially the conservative element chloride (Cl), becomes increasingly important as the number of vehicles in the state dramatically increases. With the increased number of vehicles comes a need to provide ever safer traffic-ways, which translates into ice-free roads for several months in cold climates. The increase in road salt has even led the Government of Canada to recommend the inclusion of Cl as a toxic substance because of the impact of this chemical on ground and surface waters. Associated with Cl is the anti-caking salt additive, sodium ferrocyanide, which is used commonly in Minnesota. Although not toxic itself, ferrocyanide can break down to free cyanide, which is extremely toxic at low levels. Recent data collected in Minnesota has shown that chemicals associated with salting operations (Na, Cl and cyanide) can reach very high levels in runoff from sites where salt is stored and handled, even if recommended handling procedures are followed.
Groundwater impact
The most damaging meltwater component affecting ground water appears to be the two elements associated with the most commonly used road salt - Na and Cl. The damage begins at the soil interface where Na can displace Ca and Mg and disrupt the physical structure of the soil column. Chloride can lower pH and dissociate heavy metals into more soluble and mobile forms. Although both of these chemicals can continue to migrate downward, it is mostly the Cl that presents a major threat. Much more data are needed before the complexity of the Cl threat can be fully understood. Although the threat is very real and has been documented with ground water data in many places, in other places even within the same region, the threat is variable. An example of this is presented in Issue Paper G (Appendix J) for two metro area watersheds.
Wetland, open space and biological impacts
There are scant data available on the impact of meltwater on wetland systems and associated open space areas. This information is critical when the use of “natural systems” for runoff management is increasingly promoted. Among the impacts are species shifts to less desirable species, increased toxicity to various biota, and decreased diversity. Appendix E contains a list of recommended vegetative species for use in various surface water management approaches in Minnesota. A very good resource on the topic has been produced through MPCA, entitled Plants for Stormwater Design - Species Selection for the Upper Midwest (Shaw and Schmidt, 2003).
Effects of climate change
According to University of Minnesota Professor and Extension Climatologist/Meteorologist Dr. Mark Seeley, sufficient data exist to support recently observed trends of climate change in Minnesota. These trends, as well as others collected from the global climate research community (i.e., the National Academy of Sciences “U.S. Global Change Research Programs,” the World Meteorological Organization and the United Nations “International Panel on Climate Change,” and numerous national and international universities) indicate that the following changes are likely to occur in the state:
- Warmer winters.
- Higher minimum temperatures.
- Greater annual precipitation with:
- more snowfall, but faster melting and smaller snowpacks
- more days with rain (possibly when snow present)
- Local weather less predictable and forecast less accurate
- Local weather more variable with longer periods of drought and wetness
- Local weather more severe (more “storms of the century”)
- Stormwater and flood design criteria changes to reflect new conditions more accurately
- Less annual runoff, frequent summer droughts
- Lack of ice cover or thinning of cover, decreased annual freshet (high spring flows), warmer water temps, loss of wetlands, poorer water quality
The results of this phenomenon on the character of snow accumulation and melt could be substantial in the long-term. It seems clear that snow will fall in changed patterns and that which falls will accumulate less; that snowfall terminus lines will shift northward, and upward in elevation; that the mix of ice storms and rain-on-snow will increase; that the timing and rate of snowmelt will vary from current conditions; and that the likelihood of flooding events associated with rainfall during spring melt will increase. This is a future that could also imply more chemical use to provide road safety, less chance for effective storage of snowmelt for later use, and altered annual water balances. It is also assured that any scenario for the future will include a substantial amount of uncertainty in both climatic factors and social factors as solutions to perceived and real problems are implemented.
Based on this evidence, techniques for managing stormwater under these changing conditions should be considered:
- Managers should address cold climate conditions and the pollutants associated with them.
- Higher levels of treatment (i.e., pollutant removal) for meltwater, perhaps in an SWPPP, based on land use, snow management plans, and anticipated pollutant loads of priority pollutants (sediment, chlorides, nutrients) and the waters to which they discharge should be considered.
- Implementation of a “snow management plan” should be considered.
Challenges in engineering and design
Complicating factors for cold climate design
The physical and chemical processes under way in a snowpack present an extremely complicated and variable set of phenomena. The freeze-thaw cycle and the elution of chemicals that it drives have been understood for many years, but details on the migration and management of the many chemicals of concern from the snowpack are seldom pursued by runoff managers. In 1997, the Center for Watershed Protection produced a design manual intended to address many of these problems. One of the items reported in that manual was a survey of cold climate stormwater managers asking what the challenges were that they faced. Table 9.1 is a reproduction of a table from the CWP report.
A special session was held at the 2003 Maine Cold Climate Conference during which practitioners were asked the same question. Also, a public input meeting during the development of this Manual noted the basic problems in Table 9.1 were still of concern to managers. While no magic new practices exist to treat this runoff, some adaptation of our existing approach to design and snow management could be the key to addressing this situation in cold climates.
Summary of challenges to the design of runoff management practices in cold climates (Source: Caraco and Claytor,1997)
Click here to access table
| Climatic Condition | BMP Design Challenge |
|---|---|
| Cold Temperatures |
|
| Deep Frost Line |
|
| Short Growing Season |
|
| Significant Snowfall |
|
Management approaches
Meltwater management
Special management of cold weather runoff is usually required because of the extended storage of precipitation and pollutants in catchment snowpack, the processes occurring in snowpack, and the changes in the catchment surface and transport network by snow and ice. The discharges that come from urban meltwater may cause physical, chemical, biological and combined effects in receiving waters and thereby limit their quality, ecosystems and beneficial uses.
For many years the old adage “one size fits all” was tried for the management of all runoff management. Once the effects of this approach were scrutinized, however, it became apparent that applying traditional rainfall runoff BMPs was not working for meltwater in spite of their success with rainfall. The problem is usually not the large volume resulting from a significant event, although serious flooding certainly can occur. Rather, it is that the BMPs are prevented from working as intended because of ice, cold water, highly concentrated pollution and lack of biological activity. Complications encountered in cold climates simply work against many of the commonly used warm weather BMPs, reinforcing the need for the development or adaptation (e.g. revised criteria and specifications) of existing treatment practices to better address melt runoff. Additionally, the usually poorer performance exhibited during cold weather is generally not considered when management approaches are designed because of the perceived uselessness in trying to overcome the items in Table 9.1. The problems cannot be entirely negated, but any improvement in the quantity and quality of runoff will be a step forward.
Typical results of the conditions listed above include flow by-passing and flooding, lack of reaeration in the water column, pond stratification, decreased settling and biological uptake, flushing of previously settled material, and reduced infiltration capacity.
The 5 steps for management
Step 1 - Pollution Prevention
- Pollution prevention is always the best way to manage the quality of runoff from urban and rural surfaces (see next section).
Step 2 - Infiltration
- The highly soluble and perhaps toxic “first flush” should be infiltrated to the extent possible provided the source area is not concentrated in Cl or other toxic pollutants. This can be done on-site in areas with a high degree of pervious surfaces, or perhaps routed to an area where short-term detention and infiltration can occur. For source areas high in Cl and soluble toxics or near drinking water sources, infiltration should be avoided in favor of storage and slow release once sufficient flow occurs in the receiving water to dilute the effects. Note also that snow deposits should not be located directly over a designed infiltration facility because of the possibility of clogging from debris in the snow.
Step 3 - Meltwater Storage
- Excess flow that cannot be infiltrated because of preventive (frozen) or pollution conditions should be collected in a meltwater storage area with excess capacity to hold it for the later influx of water volume and particulates. These particulates can adsorb solubles and settle, thus removing a portion of the more toxic soluble load (see Ponds section).
Step 4 - Filtration
- When fine- and medium-grained solids begin to move, settling BMPs can be incorporated starting with local application, and moving to regional storage as the need dictates. Some adaptations will be needed to incorporate storage around ice layers that might be present.
Step 5 - Housekeeping
- Much of the remaining solids are too heavy to be moved by melt so they remain near the roadside, in gutters, or in the location they were dumped as part of a snow pile, available for wash-off when spring rains come. After the snowpack has totally melted and before the first rainfall (if possible) preventive measures such as street and parking lot sweeping should be pursued. Note that Step 5 could occur after Step 1 for those communities or commercial/industrial facilities that practice cleaning activities during the winter.
Management sequence
The manner in which meltwater runs off of different contributing surfaces was previously addressed. This behavior suggests that a sequence be followed to intercept and treat variable quantities and qualities of runoff as they emerge. The following general, idealized approach should be used in planning a strategy for optimizing treatment effectiveness when it is possible to implement (see sidebar). Specific BMP adaptations to account for these strategies will be discussed later in this section.
Pollution prevention
Keeping contaminating materials away from paved surface and out of accumulated or dumped snow is the key to minimizing the pollution associated with meltwater runoff. Management approaches that help accomplish this include:
- Judicious use of de-icing and anti-skid chemicals, which then indirectly control secondary effects like heavy metal speciation and soil character changes from Cl
- Less additives like cyanide (CN) to salt
- Better chemical storage and mixing (covered storage and mix areas, mix only needed amount)
- Improved application technology with trucks, such as weather monitoring (RWIS or “road weather information systems”), direct application to roadway, and brine wetting
- Snow removal and meltwater routing to less sensitive receiving waters or treatment facilities
- Design of Cl dilution system to lower its direct impact
- Rapid sweeping as soon as snow gone from paved surfaces
- Litter control
- Erosion control
- Disconnection of impervious surfaces/reduced pavement (such as narrow roads, fewer parking spaces)
Chloride is the cause of many problems associated with snowmelt runoff. Chloride is a very soluble, conservative chemical that migrates easily through treatment systems and soil. High Cl levels decrease sorption of heavy metals and mobilize them. This leads to release of these polluting materials from storage areas with high Cl levels, as density stratification leads to the build-up of Cl to very high levels if not properly flushed from bottom waters. Methods to prevent this are discussed in the ponding section.
Infiltration
After some basic pollution prevention is practiced, the next phase of runoff management should be to soak in as much of the meltwater as possible, provided the source area does not contribute high Cl or soluble toxic pollutants.
The treatment available from infiltrating meltwater through soil (filtration, ion exchange, adsorption, and biological decomposition/transformation) will remove many of the most polluting contaminants typical of low density urban areas. These practices are, therefore, most appropriate for residential and open space areas within a watershed. Local infiltration systems, like bioretention (rain gardens, swales) and dry ponds are a good approach to route water for infiltration or filtration. All flows to infiltration practices should be pre-treated to remove particulate material that could clog the pore interstices and lead to system failure.
The problems that Cl-laden runoff can cause in both surface water and ground water were previously discussed. However, in addition to Cl, early runoff can include other soluble pollutants. The degree to which soluble contaminants will be pervasive is a function of the source load and the amount of particulates available to possibly adsorb them. Sansalone and Buchberger (1996) found that when a high level of particulate material is present in meltwater that a fair amount of adsorption occurs, negating some of the mobilization of this otherwise potentially toxic material. For source areas where runoff is a possibility, routing the runoff to a facility where an opportunity exists for this sorption to take place is a management option. Similarly, some sorption from these areas might naturally occur, so routing to a storage facility is again advisable for settling of the particulates and adsorbed material.
Although infiltration has not been a commonly used meltwater BMP in Minnesota, studies from many similar climates show that it is a feasible practice when used with precautions. Specific BMPs that involve infiltration include such practices as trenches and basins, permeable pavement and paving blocks, vegetated swales and biofilters. Criteria for infiltration are contained in the BMP sheet for infiltration practices in Chapter 12.
Routing meltwater into or away from an infiltration system is also an active meltwater management decision that can be made depending upon conditions. For example, highly Cl-laden water can be routed away from an infiltration system that might operate during three seasons, but not the winter. On the other hand, meltwater from a residential area could be routed to an infiltration system to take advantage of early melt infiltration into a dry infiltration basin.
Typical snow management techniques
Stormwater ponds
The most commonly used rainfall runoff BMP has been various versions of detention ponding. Difficulties in applying warm weather detention concepts to cold weather meltwater treatment occur with higher runoff volumes and increased pollutant loads, ice layers and frozen/sand-plugged conduits, anaerobic conditions, greatly enriched under-ice accumulation of pollutants, circulation problems and resuspension.
Figure 9.5 graphically summarizes the many processes that work to limit the effectiveness of ponding during meltwater events, including:
- Densimetric stratification caused by accumulation of road salt in runoff
- Anaerobic conditions evolving once ice prevents reaeration and baseflow ceases
- Release of pollutants, once thought to be permanently removed, from both bottom sediment and interstitial waters
- Displacement and flushing of highly polluted under-ice pond water with the first waves of meltwater that sink below the ice layer in ice-free areas near the inflow
To better understand the dynamics of sedimentation and resuspension necessary for building better cold climate detention systems in the future, and for retro-fitting the thousands in place already in Minnesota, better data collection will be needed. Such data collection should include meltwater treatment adaptations, such as seasonal detention, variable outlets, under-ice circulation, and first melt diversion into or around a treatment system.
In spite of the fact that detention systems often do not work well under typical designs in cold weather, they play a prominent role in treatment of meltwater. In the areas draining the paved areas and accumulating snow and ice near paved areas, particulate content can become extremely high because of a winter’s accumulation of anti-skid sand and urban debris. Routing this runoff to a detention facility prior to release to a receiving water is a reasonable thing to do. This large mass of particulates near these surfaces also plays an important role in adsorbing soluble pollutants that otherwise might escape further treatment. For both of these reasons, an adapted ponding system is among the list of recommended treatment methods for meltwater management.
Ponds also provide an opportunity to store, mix and slowly release pollutants mobilized during a melt event. Oftentimes, pollutants like Cl in meltwater can rise to toxic levels defined by MPCA water quality standards (Mn. Rules 7050) as beginning to show chronic toxicity at 230 mg/l. If routed into a storage facility and slowly released when sufficient water is, for example, flowing in a receiving stream, the toxic effects can be minimized. Ponds can also be used to accumulate all or a substantial part of the meltwater volume for later release when biological and physical constraints are less apparent.
It is important to recognize the potential pollution problems that Cl and toxic contaminant build-up in a pond can cause when released. A delicate balance needs to be pursued in deciding whether to adjust pond level to pass Cl-laden runoff downstream or retain as much as possible for later release when flows are higher. Retaining polluted water all winter long only to discharge it all at once in the spring is not in the best interest of receiving waters, but this is what can happen in a pond not managed for seasonally changing conditions. In no case should ponds be drained in the spring following a winter’s long accumulation of under-ice contaminants. If lowering is done, it should be done in the late fall before freeze-up.
Wetland and biological-based systems
In Minnesota, wetlands often act as modified detention facilities by virtue of their sheer numbers and the location they occupy in the drainage landscape. Most of the constraints listed above for ponds also apply to the proper operation of wetland treatment systems. In addition, however, is the sparse biological activity during the cold weather season. Vegetative uptake, filtering and microbial activity are all effective mechanisms to reduce pollution related to biological activity during warm weather that are much reduced when the weather is cold. Although sedimentation might continue to play a role in meltwater treatment, provided an ice layer does not prevent it, decomposition, chemical adsorption and biological transformation will all likely be reduced.
The impacts of Cl-laden meltwater on vegetation were previously discussed. Greatly reduced germination and growth of seeds, reduced community biomass, taxa and productivity, and a shift to less desirable species are all the effect of pollution loading to wetlands.
Other biological systems that are commonly used for rainfall runoff also suffer a drop in effectiveness in winter weather. For example, water draining to vegetated swales and bioretention (rain gardens) systems experience a drop in water quality because of reduced pollution removal.
Even though the pollutant removal effectiveness of biological systems is less during cold weather, these systems certainly have their place in an overall runoff management program. Low-lying wetlands and bioretention areas are the first place that soluble-laden first meltwater will migrate and soak into the ground. Standing vegetation, although not green and vibrant, still provides a measure of filtration as meltwater flows through. Soil microbes still live and consume nutrients even in the dead of winter. Accumulation of Cl is generally not a problem in shallow biological systems, as long as very highly concentrated levels are not routed directly to them. Even when this does occur, salt tolerant vegetation can survive. The best salt tolerant plant species for use in Minnesota are contained in Appendix E of the Manual.
Filtration, hydrodynamic structures and treatment trains
Filtration was to some degree addressed in the previous section on wetlands. Filtration also plays a role as part of a treatment train, or sequence of treatment steps designed to remove incrementally greater pollution as runoff water flows through. Filtration through a granular inorganic (sand, perlite) or organic (compost, leaf pellet) medium can be a fairly effective way to treat many of the pollutants associated with meltwater. The organic materials are less attractive in Minnesota because of the potential for phosphorus leaching into our lake dominated receiving waters.
Filtration is usually one of the last stages of a treatment train, typically preceded by processes such as screening, settling, floatable skimming, aeration, and chemical addition. Filtration is usually the final process before system infiltration or discharge to an outflow pipe connected to a storm sewer.
These systems can be particularly effective when placed as a sub-grade unit below the frostline. Sub-grade construction also allows for surface land to be used for other things, such as parking or open space.
Many new proprietary management systems are on the market today with promises of year-round effectiveness. Perhaps the most promising practices for meltwater are the treatment trains that incorporate settling, floatables skimming, and filtration through some kind of organic or synthetic media. Theoretically, these systems should be able to settle the solids associated with anti-skid grit added over the winter, then remove a fair portion of the soluble toxics also washing off in a melt. Unfortunately, conservative elements like Cl will move through these systems unchanged. The Environmental Technology Verification (ETV) program of USEPA has begun to test the claims of many proprietary units. Available results from this program are incorporated into BMP design sheets in Chapter 12, especially if field data on cold weather performance have been part of the testing procedure on the effectiveness of these systems.
Other considerations
Alternatives to sodium chloride (NaCl)
Perhaps the most vexing problem facing cold climate water managers today in many parts of the world is the accumulation of Cl from the ever-increasing application of road salt. As a conservative element, Cl moves readily through all commonly used treatment devices and into both ground and surface waters. The only effective means to remove Cl is through reverse osmosis, which does not lend itself to the large volume of runoff associated with a melt runoff event. Other treatments, like evaporation, do not work well during critical cold periods and only serve to concentrate the pollutant for later attention. The treatment approaches that seem to have some likelihood of success are:
- Wiser and less use (the focus of most transportation managers as long as safety is not compromised)
- Dilution (mix high load runoff with low load runoff)
- Detention and slow release to avoid toxic shock.
Alternative chemicals have shown some promise in the past, but each alternative seems to bring associated impacts once scrutinized. Yet, the search for, and evaluation of alternative chemicals or artificial substances for deicing or anti-icing continues. “Smart salting” is the preemptive application of deicer to prevent ice from forming (anti-icing). In Minnesota, the use of liquid MgCl2 spray on bridge decks has proven to be an effective way to avoid repeated NaCl application at high doses. Continued data collection on the presence of Cl in receiving waters is essential to the development of a reasonably protective Cl strategy. Other routinely mentioned alternatives to NaCl use are calcium chloride (CaCl2), calcium magnesium acetate (CMA), potassium formate (KFo), potassium acetate (KAc) and urea (used almost exclusively at airports). Until such time as these alternative sources are shown to be effective in controlling ice, environmentally suitable and economically affordable, NaCl will continue to be the chemical of choice by those responsible for keeping roads safe. However, Minnesota (primarily through Mn/DOT programs) will continue to explore alternatives to the use of NaCl and ways to lower the impact of salt on our receiving waters.
Winter construction season
A recent trend in Minnesota as the winters have seemed to be more mild and construction techniques improve is to continue or even initiate building during the winter. Going into a winter building season means all too often that soil and slopes are left bare all winter and exposed to snowmelt and early spring rainfall events with little protection in place. Under the Phase II NPDES permit provisions, a Stormwater Pollution Prevention Plan (SWPPP) must be produced for each construction site over one acre, but often the provisions of the SWPPP and local ordinances are ignored during the winter because of the infeasibility, for example, of getting vegetation started or of placing material over a frozen surface and having it blow away. A small amount of planning before cold conditions set in could prevent the serious erosion and pollution problems associated with these sites in the spring.
Following is a list of practices and options to consider before the cold weather construction season. Many of these elements are currently required as part of the NPDES Construction Permit, but unfortunately are often overlooked or considered infeasible during cold weather. Effective implementation of all permit requirements during cold weather is important.
- Terminate activity until warm weather returns, if construction not required over winter.
- Sequence work such that all earth-moving and soil impacting activities occur prior to freeze-up.
- Stabilize all exposed soil surfaces with vegetation, mulch or synthetic cover before the ground surface freezes and sprays become inoperable.
- Seed before October 1st to assure germination and adequate growth before cold conditions prevent growth.
- Establish stable access/egress points and stockpiling some gravel on site to maintain these routes during the winter season.
- Install roads to keep all vehicles off of exposed soil.
- Open limited new soil exposures (if any at all) and stabilize them immediately.
- Establish perimeter controls and inspect them weekly throughout the winter for structural integrity (use surface bags or rolls when posts and staples cannot be driven into the ground).
- Maintain a stockpile of sandbags and other erosion and sedimentation controls (ex. rock bags, erosion blankets) to address problems that need immediate attention.
Snow management
The plowing, relocation and collection of snow presents some very real management questions in need of support data. In most urban areas, a number of approaches are followed depending upon the level of urban density. In residential areas, snow is generally plowed to the side of the road and allowed to accumulate there all winter long. However, in commercial/industrial zones, snow is often plowed to a corner of a parking lot, and in densely-developed urban centers, snow is often removed to a totally different, often remote area, where it is dumped for an entire winter season (Figures 9.6 a and b in sidebar). Local practices seem to vary considerably based on tradition, expectations and the cost of removal operations. Assuming snow is collected, the design of “snow dumps” must take into account the fact that snow eventually melts and will need somewhere to flow, either off of the land surface or into the ground. Of particular need is data on the impact of these facilities on both ground and surface waters. Until adequate data are available, commonly accepted snow dump guidelines include:
- If possible, collect snow on an impervious pad and divert melt for treatment (ex. detention, routing to a wastewater treatment facility).
- If runoff collection and treatment is not an option, locate on a flat slope well away from surface water bodies, outside of the floodplain and well above the ground water table.
- Place the collected snow over well-drained soil to allow filtration, adsorption and microbial activity.
- Clean-up of debris left after the snowmelt and before the first spring rains fall, and restore the soil if needed.
- Monitor the quality of snowmelt and of the receiving water, especially if it is the local ground water system.
- Collecting and treating snow dump meltwater should become more important if a sensitive receiving water is at stake.
Low Impact Development (LID)
The movement in runoff management toward less structural, “low-impact” development techniques shows a great deal of promise for snowmelt management. The effectiveness of this approach to runoff management relies to a great extent on the biological and soil systems within a watershed. The ability of these systems to operate in an acceptable manner could mean they are propelled to common practice or doomed to failure. Much of the discussion in this section stresses the role that low impact approaches can have in meltwater management. Research on this topic will be entered into the BMP design sheets. Practices such as porous pavement, ground water recharge by local infiltration, use of grass swales, and road drainage infiltration systems have been found to be effective under cold climate conditions, as long as they are adequately maintained to assure their effective performance.
Planning and education
All of the design and evaluation assistance that will be contained in the Minnesota Manual will be meaningless if the results do not get properly interpreted and distributed, both to the local officials making decisions and to the public that must live with those decisions. For example, a public clamoring for ice-free roads could be in direct conflict with a reduced salt strategy. Local officials also need data and technical assistance to make good decisions on meltwater management. The Minnesota Stormwater Manual is intended to fulfill at least some of this need. Preparation of more of this kind of “on-the-ground” technical information in the hands of everyday managers is essential to improve water management in cold climate areas.
Design adaptations for cold climates
Unified sizing
How can design criteria be recommended such that everyone uses the same approach? A methodology for determining the snowmelt volume to add into the runoff calculations is suggested as input independent of whatever method is used for defining “water quality volume.” The unified sizing criteria for ponding includes adaptations that would account for less effective cold weather treatment, if possible. This may be achieved through initial design or through retrofit of existing facilities. This runoff volume should also be considered in other BMPs besides ponding and in the use of any kind of credit for meltwater design.
Water quality sizing of snowmelt
Minnesota climatology data supports a common rule of thumb that most of the snowpack disappears in the spring over a period of about ten days. The question can be raised as to why this volume should be important if BMP facilities are generally designed for treating a runoff event lasting only 24 hours. That is, why would on average 1/10 of a snowmelt runoff volume going into a facility designed to treat a much larger volume be a problem? This is a very valid comment. Clearly, if the systems are built to store a large volume of rainfall runoff, there will be no problem. The difficulty arises when complicating factors in cold weather prevent the full storage volume for a pond, or infiltration capacity of an infiltration device, or conveyance for a diversion to be available during the period of time when they are designed to operate. Suddenly snowmelt could receive less than adequate treatment or by-pass any treatment whatsoever.
Various methods to deal with the conditions experienced in cold climate BMPs were suggested previously. But the question remains, should adaptations or sizing changes be part of the recommended criteria or simply recognize the need to change our approach to get the same treatment as the facility was intended to achieve during warm weather? Should we instead think in terms of the entire snowmelt volume over ten days and compare it with the daily value used for warm weather runoff events because the treatment levels will not be the same?
This table shows recommended elements of a community snow management plan
Click here to access table
| Plan Element | Recommended Practices | |
|---|---|---|
| Formalize snow removal and disposal goals and objectives (action steps) | Adopt community snow removal and disposal goals and objectives so citizens know what to expect under certain conditions; clarify intent of wiser salt use | |
| Develop plowing and removal/redistribution plan | Formalize goals and objectives into an action plan that clearly delineates steps that will be taken under different snow and ice scenarios; target problem areas (such as ice formation) | |
| Characterization of storage areas | Identify areas where both public and private salt and salt-sand mixes are accumulated and stored, and the characteristics of these areas, such as:
|
|
| Cover exposed chemicals | Provide a small containment shelter with full coverage and a 3” lip to stop small flow from even leaving the storage pile. For larger operations, full coverage of both storage and mixing areas, and removal of unused pre-mixed sand/salt back into covered storage is recommended | |
| Identify the amount and manner of salt use | Determine the volume of salt being used, by whom, and where they are storing and applying it | |
| Identify training programs for salt handlers | Institute formal training program for all loaders and applicators | |
| Specify methods used to minimize salt application rate | Develop protocol for promotion of less salt use through driver training, equipment calibration and maintenance, pre-wetting techniques, antiicing, sand mixing | |
| Initiate record keeping system to track application rates by route and driver | Develop log system to track the amount of salt applied by each dri | |
| Map surface water drainage system and BMPs designed to handle runoff | Examine existing drainage maps to determine where chloride-laden meltwater runoff will go; identify sensitive water bodies; and assess the effectiveness of BMPs to treat snowmelt runoff | |
| Upgrade BMPs if needed | Identify problem BMPs that could be preventing adequate runoff treatment and program to manage them better | |
| Assess current practices used in your community | As part of SWPPP, are there any limits on construction or special BMPs required during the winter season | |
| List special provisions | Identify possible protective measures that could be adopted to prevent problems associated with winter conditions | |
| Assess need for construction control program | Notify new sites covered under NPDES construction permit process that they are required to address snow in a site SWPPP | |
| Assess need for MS4 program under post-construction elements | Address existing sites covered under the umbrella of the MS4 SWPPP or any re-development permitting | |
| X | ||
| Develop pollution prevention program focused on winter activities | List program elements that could be used to prevent runoff problems from occurring during the winter construction season; adopt ones most appropriate to your community | |
| Identify any measures that show effectiveness | Identify other methods that could be used, although not part of previous element | |
Water quality credits for onsite snow management
Credits for snowmelt management should be considered when management decisions need to be made. For example, if there is an approved and enforceable snow management plan for a fully developed urban commercial site that dictates plowed snow will be hauled to a suitable on-site snow storage area (e.g., pervious soils, sump area sized to certain design specifications, spring clean-up plan), then the stormwater BMPs can be sized according to the baseline (rainfall runoff) criteria. However, if the same site merely plows the snow to a corner of a parking lot and lets it enter a storm sewer that empties to a nearby pond with a thick ice cover, then maybe the applicable SWPPP needs more attention.
Another opportunity to incorporate credits could be possible if it is presumed that the snowmelt sizing approach showed that the snowmelt water quality volume (Vwq) is greater than the rainfall runoff Vwq. If this is the case, certain measures could lead to a reduction in that volume to the point where it approached or equaled the rainfall Vwq. Credits such as considering subtracting out roof areas that drain to pervious surfaces could be applied to adjust the snowmelt volumes. If chloride loads are of particular concern, a credit could be given for residential streets that have a “reduced salt” covenant. The street area could be subtracted out of the snowmelt Vwq computation.
Perhaps the level of inclusion of a snow management plan should be a function of whether a community is covered under an NPDES MS4 permit. That is, are there exemptions that should be considered such as waiving snowmelt criteria for sites outside of MS4 jurisdictions? Another example might involve waiving snowmelt criteria for direct discharges to streams where the ratio of site drainage area to upstream drainage areas is less than some fraction (e.g., 5 percent). This argument would be loosely founded on the dilution principle, which has been previously identified as one of the limited management approaches for Cl, but might not send a positive message (that is, using dilution to solve a water quality problem).
Snow management plan guidelines
Perhaps the best approach for incorporating cold climate considerations into a community stormwater plan is through a “Snow Management Plan”. This plan could be implemented a number of different ways. The most obvious would be incorporation into the SWPPPs developed by MS4 communities or as part of a construction or industrial site permit. There currently are no requirements for snow management plans in state regulations. For large-scale operational agencies, such as Mn/DOT, it could be adopted in its standards of practice. The plan could also be part of an ordinance that a community requires be applied for specific types of land use, such as commercial or multi-family buildings.
This section in part addresses the need for guidance, but not fully. It does not, for example, provide fact sheets for small landscaping companies that plow snow at commercial facilities, which could be produced as part of the MS4 technical information assistance effort.
The following elements (Table 9.2) are guidelines for the preparation of a Snow Management Plan. Each of these categorical discussions also has a recommendation(s) on the proper approach.
BMP design modifications
Another option originally proposed in the CWP Cold Climate BMP Supplement (Caraco and Claytor, 1997) is to incorporate additional storage or treatment volume into typical designs. For example, the CWP proposed the addition of an extra 25% Extended Detention (ED) storage to ponds for winter use. This approach could also be accommodated under the seasonal designs presented in this chapter. It is clear that the problems associated with the collection, routing and treatment of snowmelt runoff will continue to occur unless the shortcomings of using our warm weather techniques to treat a cold weather problem are addressed.
Preliminary considerations for design sheets based on cold climate performance
Applicability of BMPs for Cold Climate
It is necessary to look at the list of BMPs identified in Chapter 5 and assess their applicability for cold climates. Details on specific BMP design and maintenance are part of the design sheets that follow in Chapter 12.
Adaptation concepts
Each of the design sheets for the BMPs (Chapter 12) addresses adaptations needed to properly operate in cold climates. Following in this section, however, are some select summary adaptations for some of the engineered systems.
Infiltration basin/surface filter
Various options for use of infiltration and filtration are available for treating meltwater. Some of the installations are built below the frost-line (trenches, sub-grade proprietary chambers) and do not need further adaptation for the cold. Surface systems, however, do need some special consideration.
The problem with infiltration or filtration in cold weather is the ice that forms both over the top of the facility and within the soil interstices (Figure 9.7). To avoid these problems to the extent possible, the facility must be actively managed to keep it dry before it freezes in the late fall. This can be done by various methods including limiting inflow, under-drainage and surface disking. Even if the infiltration properties of an infiltration basin are marginal for melt, the storage available in the facility will provide some storage if it is dry entering the melt season. Routing the first highly soluble portions of melt to an infiltration facility provides the opportunity for soil treatment (filtration, adsorption, microbial activity) of these solubles. Figure 9.8 is a general graphic portrayal of an infiltration basin adapted for handling spring meltwater runoff. The adaptation in this graphic is a sub-drain installed to dewater the basin of any water heading into the freeze-up. This drain can be closed just prior to meltwater inflow and during the non-winter seasons to allow infiltration to continue downward. Also note that a clay liner can be added if the need to protect local ground water from infiltrating meltwater is important. If this adaptation is made, the basin is no longer an infiltration system, but instead becomes a filtration system or dry pond.
Proprietary, sub-grade infiltration systems provide an alternative to standard surface based systems. These systems, in essence, provide an insulated location for pre-treated meltwater to be stored and slowly infiltrated, or simply filtered and drained away if ground water sensitivity is an issue. The insulating value of these systems adds to their appeal as low land consumption alternatives to ponds and surface infiltration basins.
In cold climates, stormwater filtering systems need to be modified to protect the systems from freezing and frost heaving. Physical design and operational considerations to keep in mind for filtration systems are included in Chapter 12.
Note that although filtering systems are not as effective during the winter, they are often effective at treating storm events in areas where other BMPs are not practical, such as in highly urbanized regions. Thus, they may be a good design option, even if winter flows cannot be treated. It is also important to remember that these BMPs are designed for highly impervious areas. If the snow from the contributing areas is transported to another area, such as a pervious infiltration area, their performance during the winter season is less critical to obtain water quality goals.
Seasonal ponds
The difficulties of operating an effective storage and treatment pond in a cold climate were discussed previously. Problems exist with the thick ice cover (lack of reaeration, “impervious” cover for settling purposes, reduced storage volume) and under the ice (anaerobic conditions, resuspension of settled material, concentration of Cl and toxic material, dissolution and density stratification).
To overcome these difficulties, some seasonal adjustments can be made to account for winter conditions. The obvious need in this situation is to eliminate the effect of the ice layer. This layer can be up to several feet thick during a hard winter and can greatly reduce the availability of the designed storage volume. The result is usually a small amount of the initial melt diving under the ice in a somewhat pressurized manner forcing out water that might have sat stagnant all winter long. When the available capacity provided by limited uplift of the ice cover is filled, meltwater begins to flow over the top of the ice, which usually means outflow at the other end after very limited exposure to settling due to the “impervious” ice cover.
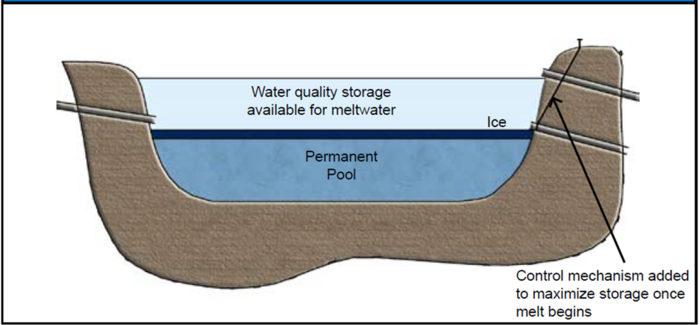
Minimizing the effect of the ice cover can be done passively through the design of surplus storage or actively through the management of water levels before ice has a chance to form and after meltwater inflow begins. The first suggested adaptation is shown in Figure 9.9. In this system, the normal design storage volumes are maintained, but a control mechanism (valve, weir, stop-log) is installed to reduce or even eliminate outflow for the normal water quality volume. This volume is then made available for meltwater, which can be held and slowly released. This approach provides for some settling time and could be used to capture high Cl flow for later slow release. The problems with under-ice build-up of anaerobic conditions and poor water quality will likely not be avoided under this adaptation.
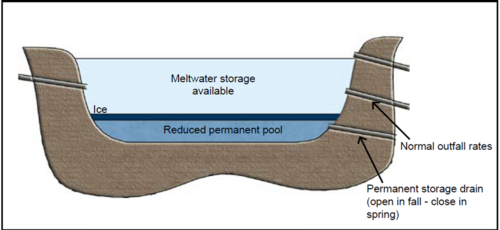
The second adaptation is one that should be used when concern over the quality of water associated with a pond is paramount. The adaptations in Figure 9.10 require more active management, but they will result in improved performance and fewer downstream water quality problems. This adaptation is especially recommended when sensitive receiving waters are a concern, or if additional treatment effectiveness is needed to achieve a TMDL requirement. The significant change made in Figure 9.10 is the addition of a controlled outlet mechanism for the permanent storage pool. Lowering this pool to a lower level will minimize the effect of an ice layer and maximize the storage available once the lower control is closed and the large spring melt occurs. The poor under-ice water quality concerns will be minimized. The “reclaimed” storage volume will equal most of the permanent pool and all of the water quality volume. The storage of all phases of the melt sequence means that solubles will be held, volume will be stored, and particulates will have a chance to both adsorb soluble pollutants and settle.
One caution for this system is that the permanent pool could completely freeze or possibly disappear entirely if the drawdown is complete. Since maintaining a healthy biological system is part of a successful detention system, it is recommended that the permanent pool not be drawn too far down such that total freeze-up or elimination occurs.
Applicability of BMPs for cold climate use
Click here to access this here
| BMP Family | BMP | Classification | Notes |
|---|---|---|---|
| PollutionPrevention | Housekeeping practices | Yes | Focus on rapid clean-up of paved surfaces after snowmelt |
| Atmospheric control | Marginal | Control of auto emissions and industrial output usually not under local control, but exposed winter soils are controllable | |
| Chemical controls | Yes | Salt management and chemical spill control can be local programs | |
| Animal waste management | Yes | Strict waste control can be covered in local ordinance | |
| Streambank stabilization | Yes | Attention to local erosion sites can reduce ice damage and sediment load from high spring flows | |
| Runoff Volume Minimization | Natural area conservation | Yes | Preserving pervious areas for meltwater to infiltrate is effective way to control volume |
| Soil amendments | Marginal | Enhancing soil permeability will increase infiltration of meltwater | |
| Reduction of impervious surface | Yes | Preserving pervious areas for meltwater to infiltrate is effective way to control volume and to minimize mobilization of pollutants | |
| Grass drainage channel | Yes | Routing meltwater over a pervious surface will yield some reduction in flow and improved water quality | |
| Rain barrel/cistern | Marginal | Capturing meltwater from a building will reduce volume but ice build-up could be a problem unless collection occurs below frostline | |
| Permeable pavement/blocks | Yes | Recent research has shown this approach to be successful in cold climates when properly installed and maintained, and when sanding kept to a minimum | |
| Soakaway pit/drywell (designed so as not to qualify as a Class V injection well) | Yes | Effective as long as system is installed below the frostline to avoid ice build-up | |
| Stormwater planter | Marginal | These are designed more for the growing season, but they do provide a sump area for runoff to collect and will infiltrate some of the volume | |
| Rooftop garden | Yes | Recent research has shown that slow melting in the spring reduces the volume running off of roof surfaces | |
| Temporary Construction Sediment Control | Preconstruction planning | Yes | Focus on sequencing to avoid open soils during winter and on limited grading prior to freeze-up |
| Resource protection | Yes | Buffers reduce runoff by providing infiltration potential | |
| Runoff control | Yes | Stable drainageways and sediment basins assure erosion control and provide storage opportunities for spring meltwater | |
| Perimeter control | Yes | These practices are especially effective during winter construction | |
| Slope stabilization | Yes | These must be installed prior to freeze-up to be effective; they must be checked often and maintained all winter | |
| Stabilized soil | Marginal | Seeding, blankets and sprayed stabilizers must all be in place and working before freeze-up; if necessary, blankets can be laid and held in place with sandbags or rock logs | |
| Inspection and maintenance | Yes | Essential for proper operation all winter | |
| Bioretention | Rain garden | Marginal | By definition, these are growing season practices, but they do provide a sump area for storage and some infiltration during a melt |
| Depressed parking islands | Yes | These can provide needed storage during the cold season and for spring runoff events; vegetation will not be a factor during winter | |
| Filtration | Media filter | Yes-to marginal | Surface systems need to be fully dry before freezeup for these to work properly; sub-grade systems can be very effective for meltwater treatment |
| Surface vegetative filter | Marginal | Vegetative filtering is reduced once vegetation dies back in the fall; some physical filtering will occur if vegetation density and depth are sufficient | |
| Combination filter | Yes-to marginal | See comments above | |
| Infiltration | Trench | Yes with caution | Effective when designed, installed and maintained properly; caution applies to limitations on source area to avoid high concentrations of Cl and toxics |
| Basin | Yes with caution | See above comment | |
| Stormwater Ponds | Forebay | Yes | Effective if designed with enough available volume to accommodate meltwater in the spring |
| Storage components | Yes | Adaptations must be made to allow meltwater runoff to receive appropriate amount of treatment (see discussion following in this section); treatment effectiveness usually lower than warm weather | |
| Outlet | Yes | Proper design of the outlet structure can be the key to ponding effectiv | |
| Constructed Wetlands | Forebay | Yes | See comment for forebay above |
| Storage components | Yes-to marginal | Volume will be less than typical pond, but provide location for storage, some infiltration, filtration and some microbial activity; biological activity at a minimum | |
| Supplemental Treatment | Proprietary sediment removal | Yes | These devices are typically installed below ground and below the frostline, and can be effective in treating sediment-laden spring runoff |
| Catch basin insert | Marginal | The location of these in a very cold location often leads to icing conditions; can be marginally effective for solids even if frozen | |
| Wet vault | Yes | See comment for proprietary devices | |
| Chemical treatment | Yes | These systems are designed to inject treatment chemicals for all flows | |
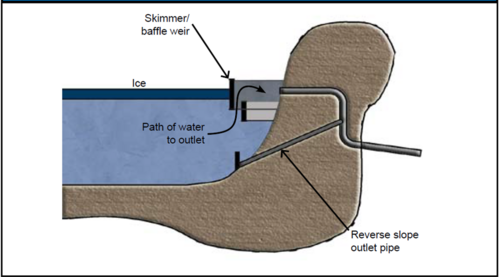
| Floatable skimmer | Yes-to-marginal | Proper installation of a floatable skimmer or baffle weir will allow water to pass even when thick ice is present; draws water from below ice layers | |
| Sorbents | Yes | These absorb chemicals usually in sub-grade systems | |
| Thermal protections | No | Do not apply to winter conditions | |
| Biological additives | Yes | See comment for chemical treatment |
The importance of baseflow, inlet and outlet design in ponds
Baseflow
The problems that develop under ice could be overcome in situations where baseflow is sufficient to keep the water refreshed enough to avoid anaerobic conditions and pollutant build-up. An assessment (in most cases a visual estimate) of the rate of inflow from baseflow expected over a winter could form the basis for establishing a drawdown level for the permanent pool. That is, the volume could be designed to be replaced on a frequency determined to avoid the depletion of oxygen and keep pollutant levels below toxic levels. Information on the source and characteristics of the inflow can also be important to pond design levels.
The total absence or occurrence of intermittent baseflow should favor a very low permanent pool level if an active management approach can be pursued.
Inlet and outlet design
One of the biggest problems associated with proper pond operation during cold weather is the freezing and clogging of inlet and outlet pipes.
Details on various outlet configurations are also suggested in the design sheets in Chapter 12. Some basic outlet concepts, however, should be mentioned. Perhaps as important as the layer of ice over the permanent pool is the blockage or hindrance of outflow from a pond because of a frozen outlet. There is a need to get water from under an ice layer to exit in a manner that does not cause splashing or gradual freezing of layer after layer of outflow. Drawing water from below the ice via a reverse sloped outlet pipe and installation of a skimmer device (baffle weir) that draws water from below the ice are two options shown in Figure 9.11.
Bioretention
Table 9.3 notes that bioretention can be of marginal effectiveness for treating meltwater because of the dormancy of the vegetation during the cold season. However, the incorporation of some sump storage into the design of any bioretention system will provide an opportunity to route and collect meltwater and begin the filtration and infiltration processes. The only adaptation then that should be needed is the incorporation of some storage as part of the system. Once relatively “warm” meltwater begins to accumulate in a bioretention system, some downward migration will likely begin and the system will activate.
Vegetated conveyance
Routing runoff over pervious drainage surfaces is a management method to promote the infiltration of water and reduce runoff volumes. Previous discussion in this paper described both the promise and the problems associated with these systems in cold weather. In essence, any infiltration should be considered an extra benefit, but the systems should not be relied upon during winter conditions to operate as well as they do during warmer weather. Some considerations to keep in mind for these systems are included in Chapter 12.
Snow and ice management
Dealing with the accumulation, removal and disposal of snow and ice is not a stand-alone BMP, but rather it encompasses many public works practices that potentially impact on the quantity and quality of meltwater runoff. Practices are as variable as the number of governmental public works departments and commercial maintenance companies providing services. Local snow removal does not usually involve collection and removal to a remote site. Rather, it is typically a matter of plowing to the side of the road or the far ends of the parking facility. Little thought is given to the fact that this snow will melt in the spring and flow into a receiving water or into a conveyance line that will flow to a receiving water.
Options for disposal of snow removed via neighborhood street and major roadway plowing are usually quite limited. The common Minnesota practice of pushing piles back from the paved surface as far as possible is encouraged. Research has shown that up to 90% of the pollution accumulated next to roadsides over the winter is deposited within about 25 feet of the road surface. Keeping the melt from this area off of the paved surface to the maximum extent possible is a positive water quality management strategy. Allowing it to soak into the ground is a good first step, followed by exposure of the melt to particulates in the roadside area so adsorption can occur.
Commercial and industrial areas that plow their parking and paved areas into big piles on top of the pavement could greatly improve the management of runoff if instead they dedicated a pervious area within their property for the snow. Even pushing the plowed snow up and over a curb onto a pervious grassed area will provide more treatment than simply allowing it to melt on a paved surface and run off into a storm sewer. (See sidebar Figures 9.12a-c)
As mentioned previously, alternatives to NaCl for road salting are not currently feasible because of cost (high relative to NaCl) and secondary environmental effects (like high BOD). Until such alternatives become available, a wise-use ethic should be the goal of every salt user. Adaptations in equipment are always being evaluated by Mn/DOT, which continually updates its statewide fleet with improved equipment. Passing down its experience and knowledge on these improvements is an important role for Mn/DOT. Adequate driver training on application methods and monitoring of driver salt use are other approaches to wiser salt use.
There has been a shift in recent years by many public works departments to reduction in anti-skid sand and greater use of salt. This shift has been propelled by the high cost of removing sand from street surfaces and stormwater conveyance and treatment systems. If this trend continues, the adverse impact of salt on Minnesota’s receiving waters is likely to increase. As difficult as sand is to deal with, it is generally inert and can be easily removed. Salt is a conservative substance that readily migrates into soil, ground water, lakes and streams, causing problems at each step along the way. A continued state program to reduce use, keep storage areas covered, educate salt handlers and improve equipment is essential to keep salt loads down as we change to greater application percentages of pure salt.
Finally, reduction in overall salt use has always been perceived as competing with driver safety. The progress made in more effective salt application techniques will hopefully be adopted by all applicators and show how the two important goals of environmental protection and driver safety can co-exist. The ultimate approach must balance safety, economics, and environmental considerations.
High sediment load
The addition of sand as an anti-skid agent to roads and parking lots can lead to the accumulation of sand in conveyance systems and pond inlets, as well as the plugging of infiltration and filtration systems. Frequent inspection of these facilities is essential, particularly in the early spring when large amounts of sand are washed from paved surfaces into runoff conveyance and treatment systems. Examining the need for clean-out of conveyance lines, dredging of forebays and ponds, and debris removal from infiltration/filtration systems should be a part of an annual inspection and maintenance program.
Many of the newly available proprietary sediment removal devices (see Chapter 12) are intended to be installed below the frostline and, therefore, operate as designed under all weather conditions. These systems come with many different design approaches, but as a group they provide a very good method of pre-treating inflow into primary runoff treatment devices during the winter and spring runoff seasons.
Secondary practices
There are some BMPs that are not generally recommended for water quantity or quality improvement because they are not as effective as other available techniques. There are situations, however, when these less used BMPs could have a possible cold climate role. One example of this is the use of dry detention ponds. These ponds have a limited long-term water quality benefit, although there is some benefit from the fact that a portion of the stormwater infiltrates while it awaits outletting. A secondary benefit could be achieved by routing overflow meltwater from a non-functioning practice into a dry detention pond to obtain even a small amount of infiltration and settling.
References
- Andrews, W.J., J.R. Stark, A.L. Fong and P.E. Hanson, 1999. Ground-water quality along a *flowpath in surficial outwash aquifer in the Upper Mississippi River Basin The influence of land use. Hydrological Science and Technology, 15(1-4):66-75. Special Issue: 4thUSA/CIS Joint Conference, November 7-10, 1999, San Francisco. American Institute of Hydrology.
- Bäckström, M., 1999. Series of Papers in Licentiate Thesis, Luleå University of Technology.
- Bäckström, M., 2002. Series of Papers in Doctorate Thesis, Luleå University of Technology.
- Buttle, J.M., 1990. Effect of suburbanization upon snowmelt runoff. Hydrol. Sci. Jour.,35(3):285-302.
- Caraco, D. and R. Claytor, 1997. Stormwater BMP Design Supplement for Cold Climates. Center for Watershed Protection, Ellicott City, Maryland, USA.
- Colbeck, S.C., 1978. Physical aspects of flow through snow. Advances in Hydroscience,11: 165-206.
- Colbeck, S.C., 1981. A simulation of the enrichment of atmospheric pollutants in snow cover runoff. Water Res. Research, 17(5): 1383-1388.
- Colbeck, S.C., 1991. The layered character of snow covers. Rev. Geophys., 29(1): 81-96.
- Granger, R.J., D.M. Gray and G.E. Dick, 1984. Snowmelt Infiltration to Frozen Soils. Canadian Journal of Earth Science, 21:669-677.
- Isabelle, P.S., L.J. Fooks, P.A. Kenny and S.D. Wilson, 1987. Effects of roadside snowmelt on wetland vegetation: An experimental study. Jour. of Envir Management, 25:57-60.
- Jeffries, D.S., 1988. Snowpack release of pollutants. National Water Research Institute (Burlington, Ontario, Canada), Report No. 88-06.
- Marsalek, J., 2003. Road salts in urban stormwater: An emerging issue in stormwater management in cold climate. In Proceedings - Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden, pp.65-74.
- Marsalek, J., G.L. Oberts, K. Exall and M. Viklander, 2003. Review of the operation of urban drainage systems in cold weather: Water quality considerations. In Proceedings - Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden, pp.1-11.
- Marsalek, P.M., W.E. Watt, J. Marsalek, and B.C. Anderson, 2000. Winter flow dynamics on an on-stream stormwater management pond. Water Qual.Res. Jour. Canada,35(3): 505-523.
- Marsh, P. and M-K. Woo, 1984. Wetting front advance and freezing of meltwater within a snow cover - 1. Observations in the Canadian Arctic. Water Res. Research,20(12): 1853-1864.
- Matheussen, B.V. and S.T. Thorolfsson, 2003. Estimation of snow-covered area for an urban catchment using image processing and neural networks. In Proceedings - Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden, pp.315-326.
- Natural Resources Conservation Service (NRCS), 1984. Engineering Field Manual for Conservation Practices. U.S. Department of Agriculture, Washington, D.C.
- Novotny, V., D.W. Smith, D.A. Kuemmel, J. Mastriano and A. Bartošová, 1999. Urban and Highway Snowmelt: Minimizing the Impact on Receiving Water. Water Environment Research Foundation (WERF), Project 94-IRM-2.
- Oberts, G.L., 2003. Cold climate BMPs: Solving the Management Puzzle. In Proceedings - Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden, pp.13-31.
- Oberts, G.L., P.J. Wotzka and J.A. Hartsoe, 1989. The Water Quality Performance of Select Urban Runoff Treatment Systems, Metropolitan Council, Publication No.590-89-062a, 170 p.
- Sansalone, J.J. and S.G. Buchberger, 1996. Characterization of metals and solids in urban highway winter snow and spring rainfall-runoff. Trans. Res. Record, 1523:147-159.
- Semadeni-Davies, A., 2003. Response surfaces for climate change impact assessments in urban areas. In Proceedings - Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden, pp.269-279.
- Shaw, D. and R. Schmidt, 2003. Plants for Stormwater Design. Minnesota Pollution Control Agency, 369 p.
- UNESCO, 2000. Urban Drainage in Specific Climates: Vol.II. Urban Drainage in Cold Climates. ed. by S. Saegrov, J. Milina and S.T. Thorolfsson. IHP-V, Technical Documents in Hydrology, No. 40, Vol. II, Paris.
- Westerström, G., 1984. Snowmelt runoff from Porsön residential area, Luleå, Sweden. In Proceedings of the Third International Conference on Urban Storm Drainage, Gothenburg, Sweden, June 4-8, 1984, pp. 315-324.
- Wheaton, S.R. and W.J. Rice, 2003. Siting, design and operational controls for snow disposal sites. In Proceedings - Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden, pp.85-95.
Annotated Bibliography
Eastern Snow Conference Series
- Research into cold climate hydrology began in earnest in the U.S. with the activities of the Eastern Snow Conference (ESC - www.easternsnow.org) in the early 1940s. This long and successful series of annual conferences continues today. This series has produced a myriad of papers that began the modern era of research into snow build-up and melt models.
The U.S. Army Cold Regions Research and Engineering Laboratory (CRREL)
- CRREL (www.crrel.usace.army.mil) also has a long history of studying the physical processes associated with snow, including the classic works of S.C. Colbeck and many others, helped to define the physics of snow accumulation and the movement of water and its contents from that snowpack after it melts. In 1966, a CRREL publication (Bates and Bilillo, 1966) noted that nearly half of the land mass in the Northern Hemisphere, and essentially everything north of 40˚N latitude can be classified as “cold regions” based upon air temperature, snow depth, ice cover and frozen ground.
International Association of Hydraulic Engineering and Research
- Meltwater runoff management has been the topic of several sessions at IAHR/IAWQ international conferences on urban storm drainage, including those in Banff in 1972, Gothenburg in 1984 and Niagara Falls in 1993. A 2005 cold climate session is planned at the August conference in Copenhagen.
International Conference on Urban Hydrology Under Wintry Conditions, March 19-21, 1990, Narvik, Norway
- Proceedings from this conference are generally not available. Gary Oberts has a set of papers delivered at the conference, but a proceedings was not published. This was the first conference dedicated strictly to cold climate hydrology and water management. It brought together researchers from Europe, Japan, Canada and the US to present ideas on meltwater management.
Center for Watershed Protection’s Stormwater BMP Design Supplement for Cold Climates (Caraco and Claytor, 1997) and revision session in Maine (2003)
- This CWP publication was the product of a workshop held in St. Paul in the fall of 1997. The document is available from CWP at www.cwp.org/cold-climates.htm
Water Environment Research Foundation’s Urban and Highway Snowmelt: Minimizing the Impact on Receiving Water, by V. Novotny, D.W. Smith, D.A. Kuemmel, J. Mastriano, and A. Bartošová, 1999. WERF Project 94-IRM-2.
- This WERF publication is a collection and synopsis of many pieces of cold climate research and data, primarily from the US and Canada. The focus is on highways, but the report is very informative on all aspects of urban cold climate runoff behavior.
UNESCO International Hydrological Programme, Urban Drainage in Specific Climates - Volume II Urban Drainage in Cold Climates, Edited by S. Saegrov, J. Milina and S.T. Thorolfsson, 2000. IHP-V, Technical Documents in Hydrology, No. 40, Vol. II.
- This UNESCO report has a decidedly European focus, but in doing so, it collects many important research summaries and indications of the current state-of-the-art on cold climate hydrology and water management.
First International Conference on Urban Drainage and Highway Runoff in Cold Climate, March 25-27, 2003, Riksgränsen, Sweden and follow-up journal series in Water Science and Technology, Vol. 48, No. 9, 2003.
- Researchers from predominantly the Scandinavian countries, Canada and the US presented a series of papers on cold climate water management. Many of the papers were published in the referenced WST periodical from 2003.
- This conference was a US version of the Riksgränsen conference referenced above. There was a markedly New England tone to the papers, but speakers from Canada and Scandinavia also presented. The CWP held a special session to receive input on revising its Cold Climate BMP Supplement, but funding for the reissuance has not been sufficient as yet. Although not all speakers prepared a paper, many are available for viewing and download at www.cascobay.usm.maine.edu/proceed.html.