Pollutant fate and transport in stormwater infiltration systems
This site is under construction. Anticipated completion date is May, 2015.
As stormwater travels across the land surface into infiltration BMPs, it can pick up various pollutants and deliver them to the subsurface. The fate and transport of these pollutants into soil, the vadose zone and ultimately groundwater depends on the type and amount of pollutant present, the volume of infiltration, the type of infiltration BMP, and subsurface conditions.
Contents
Typical stormwater pollutants
Common stormwater pollutants and their most important sources are described in the first table below. The second table provides typical pollutant concentrations in stormwater runoff. The concentrations are based on data from the International Stormwater Database.
Common pollutants of concern and sources in stormwater runoff. Adapted from USGS, 2014.
Link to this table.
| Contaminant | Contaminant source1 |
|---|---|
| Nitrogen | Naturally occurring from vegetation decomposition. Anthropogenic sources include fertilizers, farm-animal waste, faulty septic systems |
| Chloride | Salts applied to roads and parking lots during the winter. Natural sources include mineral dissolution |
| Copper | Industrial and domestic waste, mining, mineral leaching, automobile parts and fluids |
| Zinc | Industrial waste; automobile parts and fluids |
| Manganese | Found naturally in sediment and rocks. Anthropogenic sources include mining waste, industrial waste, automobile parts and fluids |
| Nickel | Naturally occurring. Anthropogenic sources include stainless steel and alloy products, mining, refining, automobile parts and fluids |
| Cadmium | Small amounts are naturally occurring. Anthropogenic sources include industrial discharge, mining waste, automobile parts and fluids |
| Chromium | Old mining operations; fossil-fuel combustion; mineral leaching; automobile parts and fluids |
| Pesticides | Residential use of lawn care products; commercial landscaping; animal wastes; municipal right-of-ways; agriculture; feedlots |
| Cyanide | Road salt; fertilizer production |
| PAHs2 | Auto emissions; elicit discharges; asphalt pavement (driveways, roadways and parking lots) with coal tar sealants3 |
| VOCs2 | Crude oil; insecticides; varnishes; paints; gasoline products; degreasers; municipal maintenance activities |
| Oil and grease | Gasoline products; plastics; dyes; rubbers; polishes; solvents; crude oil; insecticides; inks; varnishes; paints; disinfectants; paint removers; degreasers; automobile fluids |
| Microbes (including fecal coliform, E. coli, and pathogens) | Domestic sewage; animal waste; plant or soil material |
1The list of sources is for stormwater runoff only
2PAHs=polyaromatic hydrocarbons; VOCs=volatile organic compounds
3MPCA, 2014
Source: USGS, 2014, with permission
Concentrations of contaminants found in stormwater. Source: International Stormwater Database7. Because the data below are from a single source, values may differ from those contained on this page. We recommend if you are using emcs to quantify pollutant loading, you use this data instead of data from this table. Note that the table does not include information for chloride, a common pollutant in stormwater. Chloride concentrations vary seasonally and would be misrepresented in a single table. For more information on chloride concentrations in stormwater, see here.
Link to this table.
| Land use | TSS 1 | NO2 + NO3 1 | TN 1 | TP 1 | Cu 2 | Zn 2 | Ni 2 | Cd 2 | Cr 2 | CN 2,5 | Oil and grease 2 | VOCs 2,5 | Pesticides 2,4,5 | FC 3,5 | EC 3,5 | FS 3,5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Commercial | ||||||||||||||||
| Number of sites | 56 | 50 | 13 | 56 | 60 | 62 | 40 | 51 | 38 | 2 | 44 | 4 | 1 | 4 | -- | 3 |
| Number of observations | 857 | 786 | 77 | 948 | 785 | 867 | 291 | 543 | 294 | 6 | 394 | 160 | 6 | 19 | -- | 7 |
| % of samples above detection | 98.7 | 98.9 | 97.4 | 94.5 | 85 | 99.2 | 51.5 | 38.1 | 52.0 | 0 | 65.5 | 65.5 | 0 | 73.7 | -- | 100 |
| Minimum | <0.5 | <0.1 | <1.5 | <0.01 | <0.2 | <0.3 | <1 | <0.03 | <0.7 | n/a | <0.5 | <0.05 | n/a | <200 | -- | 310 |
| Maximum | 2385 | 8.2 | 18.1 | 4.27 | 569.1 | 3050.5 | 110 | 80 | 100 | n/a | 359 | n/a | 28000 | -- | 24000 | |
| Median | 52 | 0.6 | 1.75 | 0.2 | 17 | 110 | 8 | BDL6 | 4 | BDL | 5 | 0.7 | n/a | 450 | -- | 3100 |
| Industrial | ||||||||||||||||
| Number of sites | 58 | 51 | 13 | 57 | 65 | 67 | 43 | 60 | 42 | 2 | 48 | 3 | -- | 6 | -- | 4 |
| Number of observations | 619 | 536 | 85 | 638 | 569 | 627 | 300 | 525 | 312 | 9 | 370 | 144 | -- | 32 | -- | 12 |
| % samples above detection | 99.5 | 97.0 | 95.3 | 95.1 | 85.1 | 98.9 | 58.0 | 48.6 | 72.4 | 0 | 59.7 | 10.4 | -- | 90.6 | -- | 91.7 |
| Minimum | <1 | <0.02 | <1.5 | <0.02 | <0.2 | <0.5 | <2 | <0.03 | <0.7 | n/a | <0.5 | <0.05 | -- | <1 | -- | <1 |
| Maximum | 2490 | 8.4 | 15.2 | 7.9 | 1360 | 8100 | 120 | 334 | 150 | n/a | 408 | -- | 3600000 | -- | 48000 | |
| Median | 75 | 0.68 | 1.7 | 0.23 | 19 | 155 | 10 | BDL | 10 | BDL | 5 | BDL | -- | 3950 | -- | 24000 |
| Residential | ||||||||||||||||
| Number of sites | 146 | 127 | 20 | 148 | 147 | 151 | 77 | 114 | 72 | -- | 95 | 7 | 1 | 10 | 3 | 4 |
| Number of observations | 2257 | 1772 | 131 | 2380 | 1743 | 2013 | 418 | 1123 | 408 | -- | 694 | 210 | 6 | 94 | 19 | 23 |
| % of sample above detection | 99.9 | 99.0 | 98.5 | 98.2 | 86.5 | 97.0 | 42.2 | 40.4 | 48.8 | -- | 56.8 | 20.1 | 0 | 85.9 | 100 | 95.7 |
| Minimum | <0.5 | <0.03 | <1.5 | <0.01 | <0.2 | <0.5 | <0.5 | <0.03 | <0.7 | -- | <0.5 | <0.05 | n/a | <1 | 10 | <1 |
| Maximum | 4168 | 66.4 | 18.3 | 19.90 | 590 | 14700 | 100 | 70 | 70 | -- | 419 | 3.42 | n/a | 5230000 | 35000 | 200000 |
| Median | 58 | 0.60 | 2.24 | 0.26 | 11 | 69.9 | 5 | BDL | 3 | -- | 4 | BDL | BDL | 9400 | 1000 | 23500 |
| Open space | ||||||||||||||||
| Number of sites | 15 | 13 | 4 | 15 | 12 | 12 | 9 | 8 | 7 | 3 | 9 | 1 | -- | 2 | 1 | -- |
| Number of observations | 105 | 109 | 13 | 111 | 44 | 49 | 38 | 41 | 36 | 13 | 26 | 5 | -- | 6 | 5 | -- |
| % of samples above detection | 97.1 | 92.7 | 92.3 | 93.7 | 64.4 | 65.3 | 23.1 | 39.0 | 36.1 | 15.4 | 34.6 | 60.0 | -- | 100 | 100 | -- |
| Minimum | <1 | <0.1 | <0.5 | <0.01 | <0.8 | <5 | <2 | <0.04 | <0.7 | <0.01 | <1 | <0.2 | -- | 1900 | 100 | -- |
| Maximum | 4168 | 3.4 | 3.3 | 0.76 | 210 | 390 | 100 | 8 | 120 | 0.08 | 11 | 0.84 | -- | 63000 | 4700 | -- |
| Median | 58 | 0.5 | 1.1 | 0.129 | 6 | 25 | BDL | BDL | BDL | BDL | BDL | 0.77 | -- | 2150 | 1100 | -- |
| Rooftop | Water quality from rooftops varies with the type of roof. For more information see the section on Water quality considerations for stormwater and rainwater harvest and use/reuse | |||||||||||||||
TSS=total suspended solids, NO2=nitrite, NO3=nitrate, TN=total nitrogen, Cl=chloride, Cu=copper, Zn=zinc, Ni=nickel, Cd=cadmium, Cr=chromium, CN=cyanide, VOC=volatile organic compound, FC=fecal coliform, EC=E. coli, FS=fecal streptococci
1 Concentrations are in milligrams per liter
2 Concentrations are in micrograms per liter
3 Concentrations are in Number per 100 milliliters
4Data is for trans-1,3-Dichloropropene and bromomethane
5 Data was selected from states with a similar climate to MN. The appropriate states were determined using Figure 1.3 from the Stormwater BMP Design Supplement for Cold Climates document.
6BDL = below detection level
7The following censoring techniques were used for this data:
- If detection rates were 90% or greater, a value of "0" was substituted for non-detects.
- If detection rates were greater than 50% but 90% or less, a value of 1/2 the detection limit was substituted for non-detects.
- If detection rates were 50% or less, the median was assumed to be below the method detection level.
Nitrogen
| Summary of characteristics of nitrate-nitrogen. Sources:Pitt et al., 1994, 1999; Weiss et al., 2008; ATSDR, 2011. | |
| Mobility | Mobile |
| Solubility | High |
| Abundance in stormwater | Low/moderate |
| Toxicity | Low. Primary concern is for infants less than 6 months in age. |
| Degradation potential | High in anaerobic environments; low in aerobic environments |
| Adsorption/absorption | Low |
| Plant uptake | High |
| Potential risk to groundwater | Low/moderate based on high mobility but relatively low concentrations in urban stormwater. |
For an excellent review of nitrogen, link here. The following discussion provides a general overview of nitrogen in stormwater and fate and transport in soil and the vadose zone.
Nitrogen is found in many forms in stormwater runoff, with the most common forms being ammonium+ammonia, organic nitrogen, nitrate, and nitrite. Detectable concentrations of nitrogen occur in more than 95 percent of samples collected from urban runoff (International Stormwater Database). Total nitrogen concentrations in urban stormwater are typically in the 1 to 2 milligram per liter range. Concentrations tend to be somewhat higher in residential areas compared to other land uses (International Stormwater Database).
Ammonium, ammonia, and organic nitrogen comprise the reduced forms of nitrogen and typically account for about two-thirds of total nitrogen in stormwater runoff, although this varies widely with source area (see EPA). Together, these forms are expressed as Total Kjeldahl nitrogen. These forms of nitrogen have low mobility and are attenuated in most stormwater management BMPs through adsorption or oxidation to nitrate.
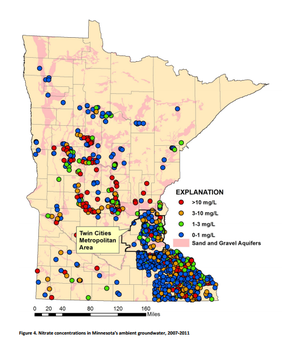
Nitrate is highly mobile in aerobic environments. It is estimated to be the most common nonpoint-source groundwater contaminant in the world (Gurdak & Qi, 2012). Despite its high solubility, nitrite is detected with much less frequency than nitrate because nitrite oxidizes rapidly to form nitrate. Nitrate concentrations in stormwater are typically 1 milligram per liter or less, well below the drinking water standard of 10 milligrams per liter.
Ammonia is highly toxic to humans and aquatic organisms, but concentrations in stormwater are typically well below levels of concern. Nitrate has relatively low toxicity, although concentrations exceeding 10 milligrams per liter in drinking water can lead to the phenomenon known as “blue baby syndrome” which affects babies less than 6 months old (Prey et al., 2000). Nitrates and nitrites have not been classified as carcinogenic, however a metabolic pathway exists that lead to formation of N-nitroso compounds, some of which are carcinogenic (ATSDR, 2011).
Chloride
| Summary of characteristics of chloride. Sources:Pitt et al.; Neiber et al., 2014. | |
| Mobility | Mobile |
| Solubility | High |
| Abundance in stormwater | Seasonal high (winter, early spring) |
| Toxicity | Low |
| Degradation potential | Low |
| Adsorption/absorption | Low |
| Plant uptake | Low |
| Potential risk to groundwater | High |
Chloride in stormwater often comes from the salts used in road surface deicing agents. Only limited data was found in the International Stormwater Database for chloride concentrations in stormwater runoff. Chloride was detected in approximately 38 percent of the 29 samples that were submitted to the database. Only sites in northern climates were included in this analysis.
Chloride is highly mobile in soil and will readily leach through the vadose zone and into groundwater.
At elevated concentrations, chloride can become toxic to aquatic life. Elevated levels of chloride can also result in low oxygen conditions, leading to the release of phosphorous and metals sorbed to the solids (Novotny et al., 2008). In addition, high levels of chloride will increase the density of the water, causing the salt containing water to settle to the bottom of the water body. This results in stratification and disrupts lake mixing patterns (New Hampshire Department of Environmental Services).
Cyanide
| Summary of characteristics of cyanide. Sources:ATSDR, 2006; EPA Technical Factsheet: Cyanide, N.D. | |
| Mobility | Mobile |
| Solubility | Depends on form of cyanide. |
| Abundance in stormwater | Seasonal (highest in winter, early spring) |
| Toxicity | High |
| Degradation potential | Moderate if not at toxic concentrations |
| Adsorption/absorption | Nitriles and soluble cyanides (e.g. H- and K-cyanide) have low absorption potential. Insoluble forms may sorb to soil particles. |
| Plant uptake | Low |
| Potential risk to groundwater | Low based on low concentrations in stormwater runoff |
Cyanide is often found in road salt, where it is used as an anti-caking agent. Another source of cyanide is discharge from industrial facilities. Only limited data was found in the International Stormwater Database for cyanide concentrations in stormwater runoff. Cyanide was detected in approximately 10 percent of the 23 samples submitted to the Database. As with chloride, only sites in northern climates were included.
Mobility in soil depends on the form of cyanide. Nitriles have the potential to leach to ground water as they do not adsorb to soil. They tend to be resistant to hydrolysis in soil or water. Cyanide-containing herbicides have more moderate potential for leaching. Soluble cyanide compounds such as hydrogen and potassium cyanide have low adsorption to soils with high pH, high carbonate and low clay content. At pH less than 9.2, most free cyanide is expected to convert to hydrogen cyanide, which is highly volatile. Soluble cyanides are not expected to bioconcentrate. Insoluble cyanide compounds such as the copper and silver salts may adsorb to soils and sediments EPA.
Cyanide is an extremely toxic pollutant. It prevents the body from using oxygen and at a sufficient concentration it can lead to death. Low exposure can cause headache or dizziness (ATSDR, 2006). Chronic exposure can lead to nerve damage or thyroid problems.
Metals
| Summary of characteristics of metals. Sources:Pitt et al., 1994; Weiss et al., 2008; ATSDR, 2004, 2005, 2007, 2012 | |
| Mobility | Very low/intermediate |
| Solubility | Low |
| Abundance in stormwater | Low/high |
| Toxicity | Variable (see discussion in text) |
| Degradation potential | Low |
| Adsorption/absorption | High |
| Plant uptake | Low |
| Potential risk to groundwater | Low, except possibly for zinc |
A nationwide analysis of stormwater runoff by Pitt et al. (2004) found one or more metal in almost all samples tested. Of primary concern are cadmium, copper, lead, and zinc (Nieber et al., 2014). The aforementioned metals were detected at varying frequencies in the sites that were reported in the International Stormwater Database. Cadmium was detected in 42 percent of 2,234 samples, copper in 86 percent of 3,125 samples, lead in 75 percent of 2,667 samples, and zinc in 98 percent of 3,552 samples.
Metals typically have low solubility and are not mobile in soil. Most are readily adsorbed within typical pH ranges found in soil. Mobility typically increases for most metals as pH decreases. metals may also form complexes with organic matter and these may increase their mobility.
At trace concentrations, several of these metals are essential to human life. At higher concentrations they can be toxic. Cadmium has the potential to bioaccumulate in the ecosystem, and at high enough concentrations, it can kill aquatic life. In humans, cadmium has been found to lead to kidney damage (ATSDR, 2012). Copper is toxic to both human and animal life. Short term exposure often results in gastrointestinal distress while long term exposure can lead to kidney damage. People with Wilson’s Disease are especially vulnerable to the effects of copper (ATSDR, 2004). Lead toxicity targets the nervous system and long term exposure may result in a decreased performance in tests that measure the function of the nervous system. Lead can also result in anemia or a small increase in blood pressure. At high concentrations, lead can damage the brain and kidneys (ATSDR, 2007). Zinc is toxic to aquatic life and can effect human health if it is ingested at levels 10 to 15 times higher than the amount needed for general health. Zinc can cause stomach cramps, nausea, and vomiting. Long term exposure can lead to anemia and a decrease in good cholesterol. Zinc may also have an effect on reproduction, though this has not been confirmed (ATSDR, 2005).
Pesticides
| Summary of characteristics of pesticides. Sources: Pitt et al., 1994 | |
| Mobility | Intermediate/mobile |
| Solubility | Variable |
| Abundance in stormwater | Low/moderate |
| Toxicity | Generally high |
| Degradation potential | Variable. Ranges from days to years. |
| Adsorption/absorption | Variable; generally moderate/high |
| Plant uptake | Herbicides are more likely to be taken up by plants than insecticides |
| Potential risk to groundwater | Variable, mainly low to moderate. Fungicides and nematocides are the most mobile (Pitt et al., 1999). See Tables 2 and 3 in Pitt et al. (1999) for the mobility potential of several common pesticides. |
Pesticides in stormwater runoff come from land application of insecticides, herbicides, fungicides, rodenticides, and algaecides. Only limited data was found for pesticides in the International Stormwater Database. Pesticides were not detected in any of the 12 samples submitted to the Database. In a summary of studies and sampling events presented in Pitt et al., (1994), pesticides such as diazionon, Malathion, 2,4-D, fungicides, dacthal, as well as many others were detected in stormwater runoff. A 1990 study by the EPA found 46 pesticides in the groundwater of 35 states. In Minnesota specifically, 14 types of pesticides were detected (Pitt et al., 1994).
Pesticides have been linked to cancer, birth defects, nerve damage, and many other disorders. Toxicity varies widely among pesticides, with insecticides typically being more toxic than herbicides. Oklahoma State University produced a report summarizing toxicity for major pesticides.
Polyaromatic hydrocarbons (PAHs)
| Summary of characteristics of pesticides. Sources: Pitt et al., 1994 | |
| Mobility | Low/intermediate; decreases with increased molecular weight |
| Solubility | Low/intermediate; decreases with increased molecular weight |
| Abundance in stormwater | Varies |
| Toxicity | Generally high |
| Degradation potential | Variable. The potential for degradation depends on the types of microorganisms present in the soil as well as they type of contaminant. See Haritash and Kaushile (2009) for a review of the biodegradation process. |
| Adsorption/absorption | High |
| Plant uptake | Low |
| Potential risk to groundwater | PAHs with the lower molecular weight have a moderate to high transportation potential while the heavier PAH have a low potential. See Tables 2 and 3 in Pitt et al. (1999) for the mobility potential of several common pesticides. |