Environmental impacts of road salt and other de-icing chemicals
This page is under development. Anticipated completion is March, 2016.
Several different types of deicing chemicals exist. Those covered this section include chloride-based deicers, acetate-based deicers, and carbohydrates. A list of the chemicals approved for use by the MNDOT can be found here.
Chloride-based de-icers
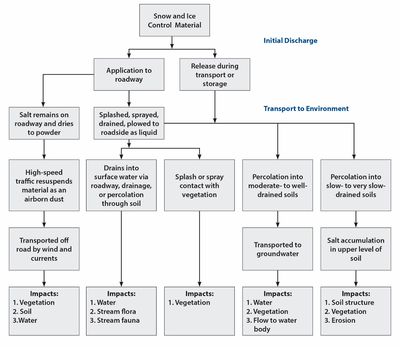
The chloride-based deicers discussed in this section are sodium chloride (NaCl), magnesium chloride (MgCl2), and calcium chloride (CaCl2). Deicers can enter into the environment during storage, transport, and application. The distribution of the deicer is a complex process, an overview of which is provided in the figure to the right. When chloride deicers are dissolved in runoff, the anion and cation dissociate. The following section separately describes the environmental effects of anions (i.e. chloride) and cations (i.e. sodium, calcium, or magnesium).
Chloride
The chloride component of chloride-based deicers does not easily precipitate, is not biodegradable, is not readily involved in biological process, and does not adsorb significantly to mineral/soil surface (Levelton Consultants Ltd., 2008). As such, chloride is highly mobile and can impact the soil, vegetation, groundwater, surface water, and air. Stefan et al. (2008) found around 30 percent of the salt applied to the roads in the TCMA makes its way to the Mississippi River. This suggests that the remaining 70 percent is either blown away, transported into the ground water, or stays within the soil, lakes, or wetlands.
Soil
Deicers reach the soil via runoff, splashing, spraying, or plowing. In general, chloride concentrations are the greatest within 2 to 3 meters from the road edge (Berthouex and Prior, 1968). Others, such as Norrstrom and Bergstedt (2001) have found salts as far out as 10 meters from the road edge, with the highest concentration within 6 meters. The distance that the salts will be transported through the soils depends on subsurface conditions. Long-term accumulation of chloride can result in reduced soil permeability and fertility, as well as increased soil alkalinity and density. As a result, there could be negative effects on the chemical properties of the soil and its ability to retain water, both of which are important to plant growth and erosion control (National Research Council, 1991). Another adverse effect of chloride in soil is its potential to release the metals sorbed to the soil particles (National Research Council, 1991; Amrhein et al., 1992; Backstrom et al., 2004).
Vegetation
Roadside vegetation can be negatively impacted by absorption of chloride through the plant roots, or from accumulating on the foliage and branches. The symptoms associated with salt impacts are similar to those of a drought; stunted growth, brown and falling leaves/needles, dying limbs, and premature plant depths (National Research Council, 1991). Figure 1.2 shows the browning of needles due to elevated salt levels. The effect of chloride on plants has been seen at distances of100 to 650 feet off the road (Fischel, 2001). The level of chloride that must be reached before the plant is harmed depends on the type of vegetation. Developers and planners often use salt tolerant vegetation near the road’s edge to lessen the impact of salt.