Difference between revisions of "Dissolved phosphorus in stormwater runoff - sources and management strategies"
m (→Definitions) |
|||
| Line 25: | Line 25: | ||
*[https://onlinelibrary.wiley.com/doi/full/10.1111/1752-1688.12366 Prestigiacomo et al.] found 10-20% of particulate phosphorus was bioavailable, compared to more than 90% of dissolved phosphorus being bioavailable. Bioavailable phosphorus in the particulate fraction increased somewhat with time after sampling, but never exceeded 30%. | *[https://onlinelibrary.wiley.com/doi/full/10.1111/1752-1688.12366 Prestigiacomo et al.] found 10-20% of particulate phosphorus was bioavailable, compared to more than 90% of dissolved phosphorus being bioavailable. Bioavailable phosphorus in the particulate fraction increased somewhat with time after sampling, but never exceeded 30%. | ||
*[https://www.semanticscholar.org/paper/Contribution-of-particulate-phosphorus-to-runoff-Uusitalo-Turtola/54189c9219e05e1b9c66b35a5af7799dc4d4e9a8 Uusitalo et al.] (2003) found 6-10% of particulate phosphorus was bioavailable, but that 34-56% was redox-sensitive, meaning it could become bioavailable under anoxic (reducing) conditions. Other papers corroborate these findings, indicating that a significant portion of particulate phosphorus can become bioavailable under anoxic conditions ([https://www.ncbi.nlm.nih.gov/pubmed/21235180], [https://www.researchgate.net/profile/Colin_Reynolds/publication/229477072_Phosphorus_recycling_in_lakes_Evidence_from_large_limnetic_enclosures_for_the_importance_of_shallow_sediments/links/5a1fdef2458515a4c3d4e69b/Phosphorus-recycling-in-lakes-Evidence-from-large-limnetic-enclosures-for-the-importance-of-shallow-sediments.pdf], [https://www.biogeosciences.net/14/3585/2017/bg-14-3585-2017.pdf], [https://link.springer.com/article/10.1007/BF00024902]) | *[https://www.semanticscholar.org/paper/Contribution-of-particulate-phosphorus-to-runoff-Uusitalo-Turtola/54189c9219e05e1b9c66b35a5af7799dc4d4e9a8 Uusitalo et al.] (2003) found 6-10% of particulate phosphorus was bioavailable, but that 34-56% was redox-sensitive, meaning it could become bioavailable under anoxic (reducing) conditions. Other papers corroborate these findings, indicating that a significant portion of particulate phosphorus can become bioavailable under anoxic conditions ([https://www.ncbi.nlm.nih.gov/pubmed/21235180], [https://www.researchgate.net/profile/Colin_Reynolds/publication/229477072_Phosphorus_recycling_in_lakes_Evidence_from_large_limnetic_enclosures_for_the_importance_of_shallow_sediments/links/5a1fdef2458515a4c3d4e69b/Phosphorus-recycling-in-lakes-Evidence-from-large-limnetic-enclosures-for-the-importance-of-shallow-sediments.pdf], [https://www.biogeosciences.net/14/3585/2017/bg-14-3585-2017.pdf], [https://link.springer.com/article/10.1007/BF00024902]) | ||
| + | |||
| + | ==Concentrations of dissolved phosphorus in runoff== | ||
| + | There are insufficient data to support recommended event mean concentrations (emcs) of dissolved phosphorus for different land uses. The following table provides a summary of data we felt is appropriate for selecting an emc for dissolved phosphorus. | ||
| + | |||
| + | {{:Event mean concentrations for dissolved phosphorus}} | ||
| + | |||
| + | ==Ratios of dissolved to total phosphorus in runoff== | ||
| + | Another consideration is the fraction or percent of total phosphorus in runoff that is in dissolved form. A more complete discussion of this [https://stormwater.pca.state.mn.us/index.php?title=Event_mean_concentrations_of_total_and_dissolved_phosphorus_in_stormwater_runoff#Ratios_of_particulate_to_dissolved_phosphorus is found here], including literature references. The data on phosphorus fractionation is limited, but the following general statements can be made. | ||
| + | *Dissolved phosphorus in urban runoff in Minnesota typically ranges from 25 to 50 percent of total phosphorus in runoff. | ||
| + | *The percent of phosphorus in dissolved form increases when phosphorus contributions from organic sources increase. For example, areas with higher tree canopy cover have a higher percent of dissolved phosphorus in runoff than areas with low canopy cover. | ||
| + | *Orthophosphate typically comprises about 60 to 80 percent of the dissolved phosphorus fraction. | ||
| + | *The percent of total phosphorus that is in dissolved form varies seasonally. Dissolved phosphorus comprises a higher fraction of total phosphorus when organic inputs are freshest, such as spring fruit drop from trees and during leaf drop in fall. | ||
==Sources of dissolved phosphorus in runoff== | ==Sources of dissolved phosphorus in runoff== | ||
| + | |||
| + | ==Management strategies for dissolved phosphorus== | ||
| + | {| class="wikitable" style="float:right; border:3px; border-style:solid; border-color:#FF0000; margin-left: 10px; width:600px;" | ||
| + | |- | ||
| + | | colspan="3" style="text-align: center;"| '''Effectiveness of stormwater BMPs in treating dissolved phosphorus (DP)''' | ||
| + | |- | ||
| + | | '''BMP''' | ||
| + | | '''Effectiveness''' | ||
| + | | '''Comment''' | ||
| + | |- | ||
| + | | Infiltration practices | ||
| + | | Effective | ||
| + | | DP may be transported to groundwater, but this generally represents a low risk to aquatic environments | ||
| + | |- | ||
| + | | Biofiltration (includes tree trenches) | ||
| + | | Ineffective | ||
| + | | Some DP removal occurs through plant uptake but phosphorus is typically released from the media | ||
| + | |- | ||
| + | | Enhanced biofiltration | ||
| + | | Effective | ||
| + | | In properly designed and maintained systems, iron, aluminum, and calcium adsorb DP | ||
| + | |- | ||
| + | | Swales designed for filtration | ||
| + | | Ineffective | ||
| + | | Addition of engineered media with a low phosphorus concentration may enhance removal through infiltration and biological uptake | ||
| + | |- | ||
| + | | Constructed ponds | ||
| + | | Limited | ||
| + | | Some biological uptake may occur, but as sediment builds in ponds, DP release may occur | ||
| + | |- | ||
| + | | Constructed wetlands | ||
| + | | Limited | ||
| + | | Some biological uptake and immobilization in sediment may occur | ||
| + | |- | ||
| + | | Green roofs | ||
| + | | Ineffective | ||
| + | | Typically leach DP from engineered media during the first several years after construction | ||
| + | |- | ||
| + | | Street sweeping | ||
| + | | Effective | ||
| + | | Most effective when done at times when coarse organic particles (e.g. from leaves) are targted | ||
| + | |- | ||
| + | | Pollution prevention | ||
| + | | Effective | ||
| + | | Focus on organic sources (e.g. yard debris), animal waste, detergents, fertilizer | ||
| + | |} | ||
Revision as of 13:31, 1 October 2021
This page is in development
This page provides a discussion of dissolved phosphorus in stormwater runoff, its sources, and strategies for managing dissolved phosphorus. While the focus is on urban runoff, the basic principles are applicable to agricultural runoff.
Contents
Definitions
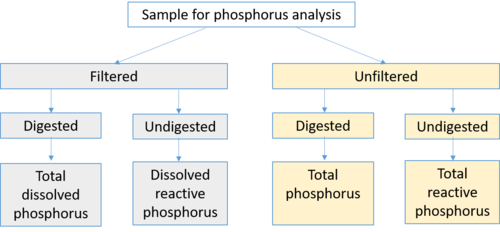
Phosphorus in water is often classified as dissolved (soluble) or particulate (attached to or a component of particulate matter) phosphorus. This nomenclature is somewhat ambiguous, however, as dissolved phosphorus consists of multiple forms of phosphorus, including phosphorus attached to other materials.
- Dissolved phosphorus is typically identified as phosphorus passing through a 0.45 micron filter. It is this dissolved fraction that is considered to be most bioavailable and most difficult to treat.
- Reactive phosphorus is the phosphorus associated with the test for orthophosphate. It consists mostly of orthophosphate but includes a small fraction of other forms.
- Soluble reactive phosphorus is a measure of orthophosphate, the filterable (soluble, inorganic) fraction of phosphorus, the form directly taken up by plant cells.
- Bioavailable phosphorus is the sum of immediately available phosphorus, which can be transformed into an available form by naturally occurring processes.
References for phosphorus forms and testing includes the following.
Bioavailability of different forms of phosphorus
Dissolved phosphorus is considered to be more bioavailable than particulate forms of phosphorus. Below is a summary of some studies on bioavailability of phosphorus.
- About 95% of dissolved phosphorus transported to Lake Erie is bioavailable to algae, while only about 30% of the particulate phosphorus attached to eroded sediment is bioavailable (Lake Erie Algae).
- Ellison and Brett (2006) found on average only 20% of the particulate phosphorus transported in runoff from urban settings was biologically available.
- Abell and Hamilton (2012) found that about 25% of particulate phosphorus in a stream dominated by stormwater runoff was bioavailable.
- Prestigiacomo et al. found 10-20% of particulate phosphorus was bioavailable, compared to more than 90% of dissolved phosphorus being bioavailable. Bioavailable phosphorus in the particulate fraction increased somewhat with time after sampling, but never exceeded 30%.
- Uusitalo et al. (2003) found 6-10% of particulate phosphorus was bioavailable, but that 34-56% was redox-sensitive, meaning it could become bioavailable under anoxic (reducing) conditions. Other papers corroborate these findings, indicating that a significant portion of particulate phosphorus can become bioavailable under anoxic conditions ([1], [2], [3], [4])
Concentrations of dissolved phosphorus in runoff
There are insufficient data to support recommended event mean concentrations (emcs) of dissolved phosphorus for different land uses. The following table provides a summary of data we felt is appropriate for selecting an emc for dissolved phosphorus.
Summary of dissolved phosphorus event mean concentrations from various studies. There is inadequate information to provide recommended emcs for different land uses.
Link to this table
| Study | Land cover/land use | Range (mg/L) | Mean | Median | Number of samples |
|---|---|---|---|---|---|
| Dallas-Fort Worth1 | Commercial | 0.01-0.47 | 0.09 | 0.06 | 42 |
| Dallas-Fort Worth | Industrial | 0.03-0.45 | 0.14 | 0.09 | 63 |
| Dallas-Fort Worth | Residential | 0.04-0.84 | 0.25 | 0.21 | 77 |
| Forth Worth2 | Transportation | 0.11 | 28 | ||
| Twin Cities3 | Mixed | 0.01-1.4 | 0.2 | 0.15 | 147 |
| Madison4 | Medium density residential | 0.52 | 0.61 | 25 | |
| Madison4 | Medium density residential | 0.4 | 0.14 | 25 | |
| Madison4 | Medium density residential | 0.14 | 0.04 | 25 | |
| Madison4 | Medium density residential | 0.05 | 0.03 | 25 | |
| Madison4 | Medium density residential | 0.04 | 0.02 | 25 | |
| Madison4 | Medium density residential | 0.03 | 0.02 | 25 | |
| Madison4 | Medium density residential | 0.04 | 0.02 | 25 | |
| Madison4 | Medium density residential | 1.54 | 0.81 | 25 | |
| Madison4 | Medium density residential | 0.12 | 0.08 | 25 | |
| Madison4 | Medium density residential | 0.11 | 0.07 | 25 | |
| Madison4 | Medium density residential | 0.11 | 0.07 | 25 | |
| US EPA Nurp Study5 | Residential | 0.143 | |||
| US EPA Nurp Study5 | Mixed | 0.056 | |||
| US EPA Nurp Study5 | Commercial | 0.08 | |||
| US EPA Nurp Study5 | Open | 0.026 | |||
| New York6 | Residential | 0.20 | 738 | ||
| New York6 | Commercial | 0.18 | 323 | ||
| New York6 | Industrial | 0.16 | 325 | ||
| New York6 | Open | 0.16 | 44 | ||
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.888 | 0.073 | 0.052 | 89 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.565 | 0.108 | 0.087 | 120 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.506 | 0.074 | 0.059 | 112 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.361 | 0.073 | 0.053 | 121 |
| Capitol Region Watershed District7 | Mixed | 0.005 -- 0.182 | 0.019 | 0.012 | 195 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.758 | 0.102 | 0.072 | 69 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 1.10 | 0.072 | 0.053 | 115 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.60 | 0.099 | 0.057 | 113 |
| Capitol Region Watershed District7 | Mixed | 0.020 - 0.499 | 0.071 | 0.046 | 138 |
1Urban Stormwater Quality, Event-Mean Concentrations, and Estimates of Stormwater Pollutant Loads, Dallas-Fort Worth Area, Texas. 1992–93 Stanley Baldys III, T.H. Raines, B.L. Mansfield, and J.T. Sandlin U.S. Geological Survey Water-Resources Investigations Report 98–4158.
2Computed and Estimated Pollutant Loads, West Fork Trinity River, Fort Worth, Texas, 1997. United States Geological survey. Water Resources Investigations Report 01–4253
3Brezonik and stadelman. 2002. Analysis and predictive models of stormwater runoff volumes, loads, and pollutant concentrations from watersheds in the Twin Cities metropolitan area, Minnesota, USA. Water Research Volume 36, Issue 7, Pages 1743-1757
457.Waschbusch, R.J., W.R. Selbig, and R.T. Bannerman. 1999. Sources of phosphorus and street dirt from Two Urban Residential Basins in Madison, Wisconsin, 1994-95. USGS Water-Resources Investigation Report 99-4021
5U.S. EPA. Results of the Nationwide Urban Runoff Program. 1983. Volume I: Final Report. PB84-185552
6New York State Department of Environmental Conservation. August 2003. Stormwater Management Design Manual. Chapter 5 - Acceptable Stormwater Management Practices.
7Outfall monitoring data for Villa Park, Trout Brook East, Trout Brook West, Trout Brook Outlet, St. Anthony, Phalen Creek, Como 3, Como 7, and East Kittsendale
Ratios of dissolved to total phosphorus in runoff
Another consideration is the fraction or percent of total phosphorus in runoff that is in dissolved form. A more complete discussion of this is found here, including literature references. The data on phosphorus fractionation is limited, but the following general statements can be made.
- Dissolved phosphorus in urban runoff in Minnesota typically ranges from 25 to 50 percent of total phosphorus in runoff.
- The percent of phosphorus in dissolved form increases when phosphorus contributions from organic sources increase. For example, areas with higher tree canopy cover have a higher percent of dissolved phosphorus in runoff than areas with low canopy cover.
- Orthophosphate typically comprises about 60 to 80 percent of the dissolved phosphorus fraction.
- The percent of total phosphorus that is in dissolved form varies seasonally. Dissolved phosphorus comprises a higher fraction of total phosphorus when organic inputs are freshest, such as spring fruit drop from trees and during leaf drop in fall.
Sources of dissolved phosphorus in runoff
Management strategies for dissolved phosphorus
| Effectiveness of stormwater BMPs in treating dissolved phosphorus (DP) | ||
| BMP | Effectiveness | Comment |
| Infiltration practices | Effective | DP may be transported to groundwater, but this generally represents a low risk to aquatic environments |
| Biofiltration (includes tree trenches) | Ineffective | Some DP removal occurs through plant uptake but phosphorus is typically released from the media |
| Enhanced biofiltration | Effective | In properly designed and maintained systems, iron, aluminum, and calcium adsorb DP |
| Swales designed for filtration | Ineffective | Addition of engineered media with a low phosphorus concentration may enhance removal through infiltration and biological uptake |
| Constructed ponds | Limited | Some biological uptake may occur, but as sediment builds in ponds, DP release may occur |
| Constructed wetlands | Limited | Some biological uptake and immobilization in sediment may occur |
| Green roofs | Ineffective | Typically leach DP from engineered media during the first several years after construction |
| Street sweeping | Effective | Most effective when done at times when coarse organic particles (e.g. from leaves) are targted |
| Pollution prevention | Effective | Focus on organic sources (e.g. yard debris), animal waste, detergents, fertilizer |