Difference between revisions of "Environmental impacts of road salt and other de-icing chemicals"
m |
m |
||
| Line 21: | Line 21: | ||
====Groundwater==== | ====Groundwater==== | ||
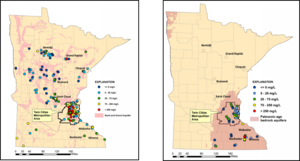
| − | [[file:Chloride in groundwater.png|300px|thumb|alt=map of chloride in groundwater|<font size=3> | + | [[file:Chloride in groundwater.png|300px|thumb|alt=map of chloride in groundwater|<font size=3>Chloride concentrations in the ambient groundwater from sand and gravel aquifers and from select Paleozoic-age bedrock aquifers in Minnesota, 2007-2011. (Reprinted from Kroening and Ferrey, 2013 with permission)</font size>]] |
Since chloride does not bind to soils, chlorides that enter the subsurface with infiltrating water may reach the groundwater table. Howard and Haynes (1993) found that 55 percent of salt applied to a catchment in Toronto enters temporary storage in shallow sub-surface waters. Cusack (n.d.) estimated that approximately 45 percent of chlorides applied as road salt will be carried to the groundwater. Chloride entering groundwater systems is likely to persist for a long time since there is no significant removal mechanism and groundwater moves slowly. | Since chloride does not bind to soils, chlorides that enter the subsurface with infiltrating water may reach the groundwater table. Howard and Haynes (1993) found that 55 percent of salt applied to a catchment in Toronto enters temporary storage in shallow sub-surface waters. Cusack (n.d.) estimated that approximately 45 percent of chlorides applied as road salt will be carried to the groundwater. Chloride entering groundwater systems is likely to persist for a long time since there is no significant removal mechanism and groundwater moves slowly. | ||
Chloride is naturally present in Minnesota due to weathering of geologic materials. , however most of the chloride found in the groundwater comes from the use of deicing salts. Kroening and Ferrey (2013) reported on the conditions of Minnesota’s groundwater from 2007 to 2011. Sand and gravel aquifers in the TCMA had chloride concentrations that ranged from less than 1 mg/L to 8,900 mg/L, with a median concentration of 86 mg/L. Approximately 27 percent of the monitoring wells located in the TMCA sand and gravel aquifers were above the secondary Maximum Contaminant Level (MCL) of 250 milligrams per liter. Statewide the median chloride levels in sand and gravel aquifers was 17 mg/L, with only 1 percent of the monitoring wells showing chloride levels above the MCL. In bedrock aquifers, chloride concentrations ranged from less than 0.5 mg/L to 680 mg/L, but in general did not exceed the secondary MCL of 250 mg/L. Chloride concentrations were highest in shallow groundwater, typically at depths of 30 feet or less below the ground surface. | Chloride is naturally present in Minnesota due to weathering of geologic materials. , however most of the chloride found in the groundwater comes from the use of deicing salts. Kroening and Ferrey (2013) reported on the conditions of Minnesota’s groundwater from 2007 to 2011. Sand and gravel aquifers in the TCMA had chloride concentrations that ranged from less than 1 mg/L to 8,900 mg/L, with a median concentration of 86 mg/L. Approximately 27 percent of the monitoring wells located in the TMCA sand and gravel aquifers were above the secondary Maximum Contaminant Level (MCL) of 250 milligrams per liter. Statewide the median chloride levels in sand and gravel aquifers was 17 mg/L, with only 1 percent of the monitoring wells showing chloride levels above the MCL. In bedrock aquifers, chloride concentrations ranged from less than 0.5 mg/L to 680 mg/L, but in general did not exceed the secondary MCL of 250 mg/L. Chloride concentrations were highest in shallow groundwater, typically at depths of 30 feet or less below the ground surface. | ||
| + | |||
| + | ====Surface waters==== | ||
| + | Chloride concentrations in surface waters tend to follow a seasonal distribution. Concentrations usually increase in the winter and decrease in the summer (Novotny et al., 2007). The chronic chloride pollution standard has been set at 230 mg/L and the acute standard at 860 mg/L by the MPCA in Minnesota Rules [https://www.revisor.mn.gov/rules/?id=7050 Chapters 7050] and [https://www.revisor.mn.gov/rules/?id=7052 7052]. These limits are based on the findings that chronic concentrations of 230 mg/L are harmful to aquatic life, while concentrations above the acute standards are lethal and sub-lethal to aquatic plants and invertebrates. Stefan et al. (2008) reported that to date there are no documented exceedances of the acute standard in the Twin Cities. However, there are 21 lakes, 22 streams, and 4 wetlands that are impaired for chloride. | ||
| + | |||
| + | Salt-containing water has a higher density than non-salt-containing water and will sink to the bottom of the water body. This can result in chemical stratification and disrupt the lake mixing patterns (New Hampshire Department of Environmental Service, N.D.; Novotny et al., 2007). Effects on surface waters may be minimized by the dilution of the deicers as they are transported to the surface water. Dilutions of 1:100 to 1:500 are estimated to mitigate negative impacts of the deicer (Fischel, 2001). Small ponds and slow streams are estimated to be most impacted by deicers because the likelihood of dilution and dispersion is lower in those environments (Fischel, 2001). | ||
| + | |||
| + | ====Air==== | ||
| + | A small percentage of the total applied chloride is evidenced to be transported by air. Blomqvist and Johansson (1999) found some deicing road salts can be transported by air 40m from the application site. Kelsey and Hootman (1992) found that sodium chloride was detected at a height of 49 feet (15 meters) within 220 feet (67 meters) of the highway. Kelsey and Hootman (1992) also found evidence of a positive correlation between plume height, and the travel distance of the constituent. The Connecticut DOT found road salt powder could travel as far as 300 feet laterally under heavy traffic conditions. Chloride transported by air can affect soil and surface/groundwater, but deposition on the vegetation is the primary concern (Levelton Consultants Ltd., 2008). | ||
Revision as of 15:25, 9 March 2016
This page is under development. Anticipated completion is March, 2016.
Several different types of deicing chemicals exist. Those covered this section include chloride-based deicers, acetate-based deicers, and carbohydrates. A list of the chemicals approved for use by the MNDOT can be found here.
Contents
Chloride-based de-icers
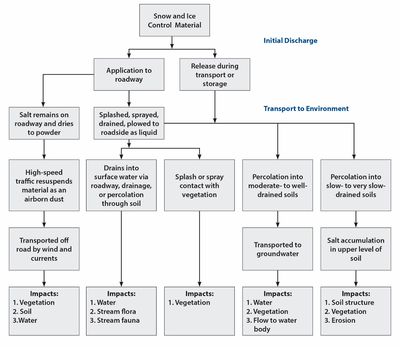
The chloride-based deicers discussed in this section are sodium chloride (NaCl), magnesium chloride (MgCl2), and calcium chloride (CaCl2). Deicers can enter into the environment during storage, transport, and application. The distribution of the deicer is a complex process, an overview of which is provided in the figure to the right. When chloride deicers are dissolved in runoff, the anion and cation dissociate. The following section separately describes the environmental effects of anions (i.e. chloride) and cations (i.e. sodium, calcium, or magnesium).
Chloride
The chloride component of chloride-based deicers does not easily precipitate, is not biodegradable, is not readily involved in biological process, and does not adsorb significantly to mineral/soil surface (Levelton Consultants Ltd., 2008). As such, chloride is highly mobile and can impact the soil, vegetation, groundwater, surface water, and air. Stefan et al. (2008) found around 30 percent of the salt applied to the roads in the TCMA makes its way to the Mississippi River. This suggests that the remaining 70 percent is either blown away, transported into the ground water, or stays within the soil, lakes, or wetlands.
Soil
Deicers reach the soil via runoff, splashing, spraying, or plowing. In general, chloride concentrations are the greatest within 2 to 3 meters from the road edge (Berthouex and Prior, 1968). Others, such as Norrstrom and Bergstedt (2001) have found salts as far out as 10 meters from the road edge, with the highest concentration within 6 meters. The distance that the salts will be transported through the soils depends on subsurface conditions. Long-term accumulation of chloride can result in reduced soil permeability and fertility, as well as increased soil alkalinity and density. As a result, there could be negative effects on the chemical properties of the soil and its ability to retain water, both of which are important to plant growth and erosion control (National Research Council, 1991). Another adverse effect of chloride in soil is its potential to release the metals sorbed to the soil particles (National Research Council, 1991; Amrhein et al., 1992; Backstrom et al., 2004).
Vegetation
Roadside vegetation can be negatively impacted by absorption of chloride through the plant roots, or from accumulating on the foliage and branches. The symptoms associated with salt impacts are similar to those of a drought; stunted growth, brown and falling leaves/needles, dying limbs, and premature plant depths (National Research Council, 1991). Figure 1.2 shows the browning of needles due to elevated salt levels. The effect of chloride on plants has been seen at distances of100 to 650 feet off the road (Fischel, 2001). The level of chloride that must be reached before the plant is harmed depends on the type of vegetation. Developers and planners often use salt tolerant vegetation near the road’s edge to lessen the impact of salt.
Groundwater
Since chloride does not bind to soils, chlorides that enter the subsurface with infiltrating water may reach the groundwater table. Howard and Haynes (1993) found that 55 percent of salt applied to a catchment in Toronto enters temporary storage in shallow sub-surface waters. Cusack (n.d.) estimated that approximately 45 percent of chlorides applied as road salt will be carried to the groundwater. Chloride entering groundwater systems is likely to persist for a long time since there is no significant removal mechanism and groundwater moves slowly.
Chloride is naturally present in Minnesota due to weathering of geologic materials. , however most of the chloride found in the groundwater comes from the use of deicing salts. Kroening and Ferrey (2013) reported on the conditions of Minnesota’s groundwater from 2007 to 2011. Sand and gravel aquifers in the TCMA had chloride concentrations that ranged from less than 1 mg/L to 8,900 mg/L, with a median concentration of 86 mg/L. Approximately 27 percent of the monitoring wells located in the TMCA sand and gravel aquifers were above the secondary Maximum Contaminant Level (MCL) of 250 milligrams per liter. Statewide the median chloride levels in sand and gravel aquifers was 17 mg/L, with only 1 percent of the monitoring wells showing chloride levels above the MCL. In bedrock aquifers, chloride concentrations ranged from less than 0.5 mg/L to 680 mg/L, but in general did not exceed the secondary MCL of 250 mg/L. Chloride concentrations were highest in shallow groundwater, typically at depths of 30 feet or less below the ground surface.
Surface waters
Chloride concentrations in surface waters tend to follow a seasonal distribution. Concentrations usually increase in the winter and decrease in the summer (Novotny et al., 2007). The chronic chloride pollution standard has been set at 230 mg/L and the acute standard at 860 mg/L by the MPCA in Minnesota Rules Chapters 7050 and 7052. These limits are based on the findings that chronic concentrations of 230 mg/L are harmful to aquatic life, while concentrations above the acute standards are lethal and sub-lethal to aquatic plants and invertebrates. Stefan et al. (2008) reported that to date there are no documented exceedances of the acute standard in the Twin Cities. However, there are 21 lakes, 22 streams, and 4 wetlands that are impaired for chloride.
Salt-containing water has a higher density than non-salt-containing water and will sink to the bottom of the water body. This can result in chemical stratification and disrupt the lake mixing patterns (New Hampshire Department of Environmental Service, N.D.; Novotny et al., 2007). Effects on surface waters may be minimized by the dilution of the deicers as they are transported to the surface water. Dilutions of 1:100 to 1:500 are estimated to mitigate negative impacts of the deicer (Fischel, 2001). Small ponds and slow streams are estimated to be most impacted by deicers because the likelihood of dilution and dispersion is lower in those environments (Fischel, 2001).
Air
A small percentage of the total applied chloride is evidenced to be transported by air. Blomqvist and Johansson (1999) found some deicing road salts can be transported by air 40m from the application site. Kelsey and Hootman (1992) found that sodium chloride was detected at a height of 49 feet (15 meters) within 220 feet (67 meters) of the highway. Kelsey and Hootman (1992) also found evidence of a positive correlation between plume height, and the travel distance of the constituent. The Connecticut DOT found road salt powder could travel as far as 300 feet laterally under heavy traffic conditions. Chloride transported by air can affect soil and surface/groundwater, but deposition on the vegetation is the primary concern (Levelton Consultants Ltd., 2008).