Spent lime, calcium water treatment residuals and application in stormwater

This page provides information on calcium in water treatment residuals and potential applications for stormwater management. The term spent lime is often used in place of calcium-water treatment residuals. Spent lime, however, includes a wide range of products resulting from the use of calcium-based materials, including many uses that render the spent lime unusable in stormwater applications (e.g. use in wastewater, for manure management, etc.). This discussion focuses only on spent lime resulting from calcium used for treatment of drinking water. While providing extensive information on water treatment residuals, there is a section focused specifically on stormwater applications for calcium water treatment residuals.
Note there are several other calcium materials that have potential application in stormwater (e.g. hydrated lime, limestone, slag). These other materials are not specifically discussed on this page except where information about the general properties and applications of calcium have applicability for stormwater management.
Contents
- 1 Overview and description
- 2 Applications in stormwater management
- 3 Production
- 4 Properties
- 5 Potential contaminants
- 6 Effects on physical and chemical properties of soil and engineered media
- 7 Effects on plant growth and microbial function
- 8 Standards and classification
- 9 Distributors
- 10 Test methods
- 11 Aging
- 12 Storage, handling, and field application
- 13 Application
- 14 Sustainability
- 15 References
Overview and description
Drinking water is often treated with lime to soften water by removing carbonate and other anions that contribute to hardness. A typical chemical reaction for softening (removing calcium carbonate hardness) is given by
Ca2+ + 2HCO3 + Ca(OH2) = 2CaCO3(s) + 2H2O
This reaction shows the formation of calcium carbonate (CaCO3). Precipitated CaCO3 is routinely referred to as spent lime. Spent lime as a hydrated slurry from water treatment is typically sent to landfills. However, lime materials precipitate out metals, perform anion exchange, and flocculate suspended and dissolved solids and may therefore have beneficial uses for pollutant removal or may have negative properties that would limit any beneficial use (Graymont; accessed 12/28/20).
Stormwater runoff from urban areas contains relatively high concentrations of phosphorus (typically 0.2-0.4 mg/L). Furthermore, engineered media used in stormwater best management practices, such as biofiltration practices, may export phosphorus due to high concentrations of organic matter in the media (See Overview of engineered (bioretention) media and applications for stormwater). This excess phosphorus negatively impacts receiving waters. Spent lime, added to engineered media in stormwater treatment practices such as bioretention, or used in engineered materials such as permeable reactive barriers, has the potential to retain phosphorus and reduce impacts to receiving waters provided it does not have other adverse effects, such as releasing contaminants associated with the treatment residual or having negative impacts to soil or vegetation.
This page provides a discussion of spent lime from water treatment, including information on its physical and chemical properties, capabilities for retaining phosphorus, and potential environmental effects. The discussion includes multiple applications in engineered media, such as use in bioretention media and permeable reactive barriers.
Applications in stormwater management
The primary use of spent lime for stormwater applications is for retention of dissolved phosphorus. Most stormwater practices are effective at retaining other pollutants that spent lime could retain, such as metals, organic chemicals, and microorganisms. Dissolved phosphorus retention in many stormwater practices has been poor, however, including biofiltration, wet ponds, sand filters, and swales.
Spent lime potentially has multiple applications for phosphorus treatment, including use in biofiltration media, in permeable reactive barriers, in sand filters, and in constructed stormwater wet ponds. Despite the potential for capturing dissolved phosphorus, however, spent lime has not been widely used in stormwater applications. Examples of stormwater applications are provided below.
- Barr Engineering developed a spent-lime treatment cell for the Ramsey-Washington Metro Watershed District to reduce phosphorus loading to Wakefield Lake, an impaired lake located in Maplewood. The treatment cell, in operation for seven years, shows that the spent lime removes phosphorus and metals to low levels (removal of 74.4 percent of ortho-phosphate and 66 percent of particulate phosphate).
- In 2018, Barr Engineering began a study to evaluate the application of spent lime to pond sediments to reduce phosphorus release during warm summer months.
- Additional projects where spent lime has been used or proposed include the following:
- Willow pond - a filtration system treating water from Willow Pond
- Lake Cornelia - a double-chamber spent lime/CC17 filter at the downstream discharge point of Swimming Pool Pond
- Inver Grove Heights spent lime reactor
- Lake Susan spent lime treatment system Spent lime reactors
- Armstrong lake spent lime filter
Calcium-based amendments, such as limestone, slag, burnt lime, and spent lime, have been used in a number of applications. However, many of these studies were not specific to phosphorus retention (e.g. sites contaminated with metals) or influent phosphorus concentrations were much higher than typically encountered in stormwater (e.g. wastewater treatment). This make extrapolation of these studies to use of spent lime for stormwater challenging. The following discussion provides a review of some literature specific to phosphorus retention by calcium materials.
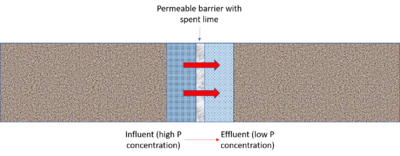
Use in permeable reactive barriers
A permeable reactive barrier (PRB) is a subsurface emplacement of reactive material(s) designed to remove dissolved pollutants as water flows through the barrier. Typically, flow through the cell occurs under a natural gradient. Treated water exits the other side of the PRB. PRBs have been widely used in groundwater applications, typically to remove organic materials or dissolved metals from contaminated water. For more specific information on PRBs, see [1], [2], [3], [4], and [5].
Materials containing lime (calcium) have been used in several applications of permeable reactive barriers. Contaminants removed by lime PRBs include phosphorus, metals, bacteria, and uranium. For stormwater, the primary chemical of concern is dissolved phosphorus. Below is a summary of studies found in the literature, including information on design and observed removal of dissolved phosphorus.
- Barr Engineering (2014) conducted a pilot study of a PRB containing spent lime. The surface area of the treatment cell was 475 square feet, with a maximum depth of 3 feet and an average width of 7.9 feet for the entire cell. The elevation difference between the lime material surface and the elevation of the riser overflow outlet was approximately 3 inches. The maximum flow through the cell was around 1.0 cubic feet per second. The treatment cell was designed to minimize short circuiting and contained approximately 35 cubic yards of spent lime material. The lime, provided directly from the City of St. Paul Water Utility after dewatering in a belt press, was placed in the cell with a backhoe. Annual maintenance consisted of removal of accumulated material on the top of the cell and mixing the top 1-foot of the lime material with a shovel. Removal over the two year monitoring period was 65.6, 63.9, and 74.4 percent for total phosphorus (TP), dissolved phosphorus (DP), and orthophosphate, respectively. Average contact times were less than 10 minutes for most of the 16 events. In designing the cell, the following considerations were made:
- Ensuring even contact between the spent lime
- Sufficient contact time between stormwater and lime to allow phosphorus removal but minimize calcium carbonate dissolution and minimize the potential that the pH of treated stormwater would exceed water quality standards
- The treatment system required enough head to facilitate rapid draining of the cell after the completion of each storm event
- Facilitate flow through the entire length of the treatment system
- Easy access for maintenance
- Baker et al. (1997; see page 697) performed column studies over a 3-year period with a PRB consisting of 50% silica sand, 45% crushed limestone, and 5% metal oxide. The columns received continuous inputs of a solution containing 3.3 mg/L P. A field scale study was also conducted with a biofilter (0.5 X 0.5 m) and wastewater applied at an average rate of 26 L/day. P removal exceeded 90 percent in both the column and field studies.
- Dunets (2014) performed column filter studies using concrete waste (CW) material and oxygen furnace slag. PVC columns (i.d. of 7.62 cm) were filled with 750 cm3 of material. Material treatments consisted of a CW/sand mixture (40% unsieved CW/60% unsieved sand, w/w), a slag/sand mixture (40% unsieved slag/60% unsieved sand), and pure sand as a control. Columns were packed at a bulk density of 1.54 g cm-3 for CW mixture and 1.59 g cm-3 for slag mixture. Greenhouse wastewater with P concentrations ranging from 20 to 60 mg/L, pH 6, were dosed at varying rates approximating hydraulic retention times (HRT) of 3 to 24 hours. Results show that greater than 99 percent P removal was achieved within 3 hours. Effluent P concentrations were correlated with pH, suggesting filter effluent pH could be monitored to approximate whether adequate treatment was being maintained, with effluent pH > 8.5 indicating effective treatment regardless of filter material or operating conditions. Some clogging was observed, primarily in the slag filter treatments. Using a larger particle size may alleviate clogging concerns but may decrease P retention. The researcher points out the Danish EPA suggests that, in order to minimize clogging, the d10 of a sediment should be 0.3-2mm, d60 0.5-8mm, and uniformity coefficient less than 4. Two additional concerns were the high pH of effluent and disposal of the treatment material.
Other studies have utilized lime in PRBs for other pollutants. For more information, see Ranjbar et al. (2017, lead), Ludwig et al. (2009, heavy metals, arsenic), Bain et al. (2006, arsenic), and Bronstein (2005, radionuclides).
There is insufficient information to provide specific design and maintenance recommendations. Based on the above studies, the following considerations seem appropriate.
- Required contact times are relatively short and prolonged inundation with water can dissolve the lime material, leading to elevated pH. Based on available information, a 10 minute contact time is recommended.
- The system should be designed to ensure contact with entire PRB system and eliminate short circuiting
- The system should be monitored for clogging. If clogging is observed, manually mechanical mixing of the material may be utilized, but limit mixing to avoid creating particles that are too fine, which could lead to clogging.
Use in biofiltration practices
Lime has been suggested as a possible amendment for removing dissolved phosphorus in biofiltration systems. There have been limited studies for this application.
- Elliott et al. (2002) incorporated calcium water treatment residuals at rates from 0.1-2.0 percent by weight into columns containing sand and biosolids as the phosphorus source. After application of one pore volume of water, phosphorus removal was greater than 95%. No negative effects on vegetation were observed. pH of the soil solution increased to 7.5, indicating the effects of lime on the pH of the media need to be considered.
- Shrestha et al. (2019) conducted field mesocosm and laboratory column studies with layered systems. The media consisted of a sand-compost mixture and an underlying layer containing 60 percent water treatment residuals and 40 percent sand. The sand-compost layer contained compost at rates varying from 20 to 100% by weight. Compared to controls, phosphorus leaching decreased by more than 50 percent in all treatments containing water treatment residuals and exceeded 90 percent at higher compost concentrations.
- Erickson et al. (2020), conducting mesocosm studies, observed that an engineered media consisting of 5% spent lime, 20% leaf compost, and 75% clean washed sand had effluent phosphate concentrations (mean= mg/L) significantly greater than influent concentrations (mean=0.197 mg/L) but significantly less than effluent from a sand-20% leaf compost treatment (mean=1.642 mg/L). The results indicate lime was not effective at preventing phosphorus leaching at the compost levels used in the study, but additional research at lower compost concentrations may be warranted.
The results suggest lime may be effective in layered systems but less effective or ineffective when mixed within the media. A concern with calcium amended biofiltration media is the potential for clogging and subsequent reduction in the hydraulic performance. Further research is needed before conclusions can be made regarding the effectiveness of lime in biofiltration systems.
Use in sand filters
Erickson (2012) conducted column studies of sand filtration treatment with combinations of C 33 sand enhanced with calcareous sand and limestone. Synthetic storm water runoff with a variable dissolved phosphorus concentration passed through the columns while the flow rate was monitored and effluent samples were tested for dissolved phosphorus concentration and pH. He observed after 48 hours, combinations of C 33 sand with limestone or calcareous sand clogged the columns and prevented them from. This is consistent with concerns for calcium-based amendments in biofiltration systems, discussed above.
Use in constructed stormwater wet ponds
Wilson et al. are currently studying the potential for spent lime to limit phosphorus release in constructed stormwater ponds. Initial results are promising and spent lime represents a more readily available material than aluminum or iron, which have historically been used to sequester phosphorus in stormwater ponds.
Production
Lime softening is a water treatment process that uses calcium hydroxide, or limewater, to soften water by removing calcium and magnesium ions. In this process, hydrated lime is added to the water to raise its pH level and precipitate the ions that cause hardness.
Quicklime and hydrated lime are frequently used in water treatment. Quicklime, known as calcium oxide (CaO), is made through the thermal decomposition of limestone or other materials containing calcium carbonate in a lime kiln. The material is heated at high temperatures, and the remains are quicklime (Meyers Emery; 2013). Hydrated lime is the result of adding water to powdered quicklime, putting it in a kiln or oven, and then pulverizing it with water. The resulting lime is called calcium hydroxide.
Whether to use quicklime or hydrated lime in a particular situation is influenced by a number of factors, such as scale of operation, method cost, transportation cost, and availability. Material cost depends on whether bagged or bulk lime, hydrated or quicklime is used. The choice between purchasing lime in bags or in bulk is a direct function of rate of use. Where chemical requirements are small, bagged lime is preferred. Conversely, at larger treatment plants it is more efficient and economical to handle bulk lime.
The following table describes characteristics of quicklime and hydrated lime.
Characteristics of quicklime and hydrated lime
Link to this table
| Parameter | Quicklime | Hydrated lime |
|---|---|---|
| Formula | CaO | Ca(OH)2 |
| Molecular weight | 56.1 | 74.1 |
| Physical state | White solid | White solid |
| Particulate size | Pulverized to lump | Powder, 200 to 400 mesh |
| Bulk density (lb/ft3) | 55 to 75 | 35 to 50 |
| Specific gravity (g/cm3) | 3.2 to 3.4 | 2.3 to 2.4 |
| Affinity for water | Reacts quickly to for Ca(OH)2 with heat of formation, 490 Btu/lb | Absorbs H2O and CO2 from air to form CaCO3 |
| Solubility | Slightly, varies inversely with temperature | Slightly, varies inversely with temperature |
| Stability in bagged storage | In multiwalled bags, max 60 days | Up to 6 months, in dry tight bags |
| pH of saturated solution | 12.4 | 12.4 |
Detailed information on the production and use of lime for water treatment can be found at the following links.
Properties
This section provides information on the physical and chemical properties of spent lime.
Physical
The following table summarizes some physical properties of spent lime.
Physical properties of spent lime.
Link to this table
| Study | Bulk density (g/cm3) | Specific gravity | pH (saturated solution) | Plastic limit (%) | Liquid limit | Ksat (cm/s) |
|---|---|---|---|---|---|---|
| EPA, 2011 | 0.68 - 1.04 | 2.35 - 3.3 | 12.4 | |||
| Elliott et al 2002; Elliott et al., 1990 | 8.93 | |||||
| Baker et al. (2005) | 0.98 - 3.43 | 189 | 295 | 1.7 X 10-5 to 1.4 X 10-7 | ||
| Birikorang | 8.43 - 11.4 |
The data on physical properties appears limited, but there is considerable information on physical properties for non-WTR spent lime. Additional information can be found in the following sources.
- INFLUENCE OF VARIOUS PRECIPITATED CALCIUM CARBONATE (PCC) “SPENT LIME” RATES ON SUGARBEET PRODUCTION, ROTATIONAL CROPS AND SOIL CHARACTERISTICS
- Hydrated lime
- Agronomic Value of Wastewater and Sugar Beet Lime Sludge Compost on Radish Crop
- Sugar beet lime sludge composts as organic fertilizers
- Lime Use in Wastewater Treatment: Design and Cost Data
Chemical
The following table summarizes chemical data for calcium water treatment residuals (WTRs). Data for calcium WTRs is limited to five studies. Data for aluminum WTRs and iron WTRs is included for comparison. The data indicate that chemical concentrations in Ca-WTRs are lower than for Al- and Fe-WTRs except for calcium, magnesium, and strontium. Soil reference values (SRVs) and soil leaching values (SLVs) are included and discussed in the next section.
Chemical properties of water treatment residuals. Values are medians for all data collected from the literature.
Link to this table
| Chemical | Type of water treatment residual | Tier 2 Soil Reference Value (mg/kg) | Soil Leaching Value (mg/kg) | |
|---|---|---|---|---|
| Aluminum and iron | Calcium | |||
| Aluminum | 51000 | 2211 | 100000 | |
| Ammonia | nd | |||
| Antimony | nd | 1 | 100 | 10.8 |
| Arsenic | 35 | 4.3 | 20 | 11.6 |
| Barium | 205 | 30.3 | 18000 | 3370 |
| Beryllium | 1.55 | nd | 230 | 5.44 |
| Boron | nd | 47000 | 124 | |
| Cadmium | 0.8 | 0.2 | 200 | 17.6 |
| Calcium | 16700 | 494367 | ||
| Carbon | 172500 | 114000 | ||
| Chromium III | 52.5 | 1.6 | 650 | 200000 |
| Copper | 48 | 2 | 9000 | 1400 |
| Iron | 51000 | 956.5 | 75000 | |
| Kjeldahl nitrogen | nd | |||
| Lead | 49.5 | 2.8 | 700 | 5400 |
| Magnesium | 2300 | 8530 | ||
| Manganese | 1950 | 73 | 8100 | 260 |
| Mercury | nd | nd | 1.4 | 6.58 |
| Molybdenum | 2 | 0.2 | 32.2 | |
| Nickel | 32.5 | 1.6 | 2500 | 352 |
| Nitrogen | 8350 | 330 | ||
| Phosphorus | 2995 | 74 | ||
| Potassium | 5550 | 846 | ||
| Selenium | nd | 1 | 1300 | 5.28 |
| Silver | nd | 1 | 1300 | 15.7 |
| Sodium | 855 | 335 | ||
| Strontium | 45 | 274 | 100000 | 5620 |
| Vanadium | 68 | 3 | 250 | 8 |
| Zinc | 183 | 5.4 | 75000 | 6010 |
nd=not detected. Reporting limits were below SRVs and SLVs.
SLV assumes 3 foot separation from groundwater and a media depth of 3 feet.
Numbers in bold exceed one or more of the risk criteria.
References: Turner et al. 2019; Elliott et al 2002; Lang, 2009; Shrestha et al, 2019; Elliott et al., 1990; 2009; EPA, 2011; Barr Engineering, 2014; Wang et al., 2014
Potential contaminants
Baker et al. (2005) summarized results of a USEPA study that examined removal of metals by lime sludge used for water softening. Removal rates, as a percent, varied with pH and are summarized in the following table.
Removal of metals by lime sludge (spent lime) used for water softening. Source: Baker et al. (2005).
Link to this table
| Chemical | Percent removal | |
|---|---|---|
| pH=9 | pH=10 | |
| Lead | >95 | >95 |
| Cadmium | >95 | >95 |
| Silver | 80 | 85 |
| Chromium III | 70 | 85 |
| Beryllium | 75 | >95 |
| Arsenic V | 40 | 75 |
| Arsenic III | 15 | 18 |
| Mercury | 20 | 55 |
| Selenium IV | 20 | 20 |
| Selenium VI; Chromium VI | negligible | |
Because of its sorptive properties, spent lime has the potential to contain chemicals that may be of environmental concern. As discussed in the previous section, median concentrations of chemicals in spent lime do not exceed Tier 2 Soil Reference Values (SRVs) or Soil Leaching Values (SLVs)(Note: Soil leaching values were calculated assuming a separation distance of 3 feet from groundwater and a media thickness of 3 feet). Concentrations of chemicals are a function of concentrations in the source water. Thus, although median concentrations are below levels of concern, source waters with elevated concentrations of specific chemicals, such as arsenic, could result in elevated concentrations in the spent lime.
Lime used for softening effectively sequesters radium from source water. Barr Engineering collected four samples for radium in spent lime, with concentrations of ND (non-detect), 0.145, 0.181, and 0.58 pCi/g. We can use information from adsorption studies to estimate likely radium concentrations in water. Although specific adsorption coefficients (Kd) were not found for spent lime, Kd values are established for a variety of geologic materials. Kd values range from approximately 100 to several thousand mL/g, depending on the geologic material. Using conservative values for Kd and the data collected by Barr, resulting concentrations in water are below the drinking standard of 5 pCi/L ([9], [10], [11]).
Barr Engineering (2014) conducted a study comparing aquatic toxicity of stormwater treated with spent lime to untreated stormwater. Two storm events were sampled and tested in a laboratory. Standard U.S. Environmental Protection Agency (EPA) methodologies were followed to test for chronic aquatic toxicity using a sensitive test species called Ceriodaphnia dubia (e.g., a zooplankton, often described as a water flea). For the May 21, 2012 test, mean young production was greater for treated stormwater than for untreated stormwater. This indicates that the treated stormwater was less toxic than the untreated stormwater. For the June 19, 2012 test, mean young production was lower for treated stormwater than for untreated stormwater. In both cases, however, the spent lime material did not produce unwanted toxic conditions in the treated stormwater. Overall, these tests suggest that the use of spent lime will not cause unintended aquatic toxicity in water receiving spent lime-treated stormwater as long as the contact time is maintained at an appropriate level.
Spent lime from water treatment does not appear to represent a concern for release of potential contaminants. If source waters have elevated concentrations of specific chemicals (e.g. arsenic, manganese), spent lime may contain elevated concentrations of these chemicals and should be sampled for comparison with SRVs and SLVs prior to reuse of the material.
Effects on physical and chemical properties of soil and engineered media
This section provides a summary of the effects of spent lime on the physical and chemical properties of soil and engineered media, including pollutant removal.
Effects on phosphorus adsorption
| Lime materials provide significant phosphorus retention. However, their performance in stormwater applications is not fully researched and there is therefore limited guidance on specific design and maintenance for stormwater applications. Case studies are discussed here. |
Phosphorus can be precipitated from a system by calcium, either through incorporation into a calcium phosphate salt, or adsorbed onto calcium carbonate. A typical reaction is given by the following.
2H3PO4+3 + CaCO3(ag) → Ca3(PO4)2(s) + 3CO3−2 + H+
2H+ + CO3−2 → H2O + CO2
There are many forms of calcium phosphate , the most stable being hydroxyapatite. Initial reactions between calcium and phosphate are very quick, with phosphate solutions coming into equilibrium with calcium carbonate within 20 minutes except at very high phosphate concentrations (5 mg/L or greater). These initial rapid reactions typically result in the formation of metastable species such as tricalcium phosphate, octacalcium phosphate, anhydrous dicalcium phosphate, and dicalcium phosphate dihydrate. The rate of formation for hydroxyapatite is very slow and results from “aging” of these intermediate precipitates (Birdsey, 1985; Clark and Turner, 1955).
Most studies in the literature examined the removal of phosphorus from wastewater or from biosolids applied to soil. Phosphate removal rates reported in the literature vary from about 60 to as high as 95 percent (Yanamadala, 2005; Birdsey, 1985; Clark and Turner, 1955; Wandruszka,2006). Factors affecting phosphorus retention include the following.
- Retention increases as surface area of the carbonate material increases
- Retention increases as the charge differential increases between the liquid solution and solid surface
- Retention by calcium decreases as iron and clay concentration increases
- Retention may increase or decrease in media with organic matter depending on the nature of the organic material
Shrestha et al. (2019) studied sorption of phosphate in bioretention media containing varying amounts of compost and amended with coir or spent lime WTRs. The study included a field-based mesocosm experiment and a laboratory study, both which assessed the effect of spent lime amendments on leachate nutrient. Spent lime significantly reduced leachate PO43− concentrations (upwards of 50%) in both the field and lab mesocosm studies compared to treatments without spent lime. While leachate P concentration increased significantly with increasing compost levels in the absence of spent lime, leachate P concentrations remained relatively uniformly low across this gradient when spent lime was added to substrate.
Elliott et al. (2002) studied phosphorus leaching in laboratory and greenhouse plots receiving biosolids application and amended with water treatment residuals (iron, aluminum, and calcium WTRs). With no WTRs, 11-21% of P leached over 4 months, compared to a loss of 2.5% for calcium-WTRs.
Barr Engineering (2014) conducted laboratory and field studies to examine phosphorus removal by spent lime. The laboratory study included column tests whereby stormwater was passed through different lime configurations. The pilot-scale field study included site identification, design, permitting, construction, and monitoring of a pilot-scale lime treatment cell. Three years of monitoring the test spent lime system showed total phosphorus removal of 60% and total dissolved phosphorus removal of 70% in this test.
Adhikari et al. (2016) observed a 76% reduction in phosphorus concentrations from water samples spiked with high concentrations of phosphorus (25 and 80 mg/L). Adsorption was 90 percent complete within 60 minutes.
In a column study DeBusk et al. (1997) observed a 42% decrease in P concentrations in stormwater effluent containing 0.40 mg/L P. Yanamadala (2005), studying the effects of lime in aquatic systems, observed that calcium carbonate was highly successful (p < 0.0001) in decreasing phosphates from 1.5 ppm, on average, to 0.4 ppm, an average decrease of approximately 70%.
Effects on physical and hydraulic properties
| Incorporated into soil, lime generally improves physical characteristics of acidic soils. However, lime may reduce hydraulic performance by producing cementitious compounds. Periodic mechanical mixing may alleviate this effect. |
Lang observed that freeze thaw cycles caused the reduction of compressive strength for both natural soils and soils treated by lime sludge but that lime sludge treated soil specimens have much higher strength than natural soil specimens even after freeze-thaw cycles. These observations point to the positive effects of lime sludge treatment in improving the soil mechanical performance properties as well as improving the durability under freeze-thaw cycles. Baker et al. (2005) observed that lime-amended soils exhibited similar soil bulk densities and increased water-holding capacity compared to non-amended soils.
A number of works on lime treatment showed there are two distinct processes that take place when lime is added in wet soil: modification and stabilization (Sherwood, 1993; Rogers and Glendinning, 1996; Boardman et al., 2001). The modification corresponds to a cation exchange process where the calcium ions (Ca2+) from hydrated lime migrate to the surface of clay particles and displace water and other ions. This process lasts for a few hours depending on the clay mineral involved, and the soil becomes friable and granular. Stabilization refers to the pozzolanic reaction which occurs more slowly over a long period of time and depends on temperature, soil chemistry and mineralogy (Hunter, 1988; Wild et al., 1993). During this process, the high pH value in soil causes silica and alumina to be dissolved and to combine with calcium producing cementitious compounds, calcium silicate hydrates (CSH) and calcium aluminate hydrates (CAH) (Choquette et al., 1987; Locat et al., 1996).
Barr Engineering observed the hydraulic conductivity of spent lime is high even when wet and does not readily clog. Other studies, however, have observed clogging in column studies with lime (Erickson et al., 2007). Gao et al. (2018) observed lime additions up to 3 percent increased hydraulic conductivity, while additions of 3-9 percent decreased hydraulic conductivity in a silty loess soil. Bhaskar et al. (2019) observed decreases in saturated and unsaturated hydraulic conductivity with addition of lime to a clay soil. Elkady et al. (2016) observed increases in hydraulic conductivity when lime was added to compacted clay soils. Nguyen et al. (2015) observed increased hydraulic conductivity after 28 days in a lime-amended silt soil, but then a decrease after 90 days, corresponding to the modification and stabilization processes discussed above. Di Santi et al. (2020) observed increased hydraulic conductivity and improved soil structure in a 7% lime-amended compacted clay soil.
These studies indicate a need for additional research on the hydraulic effects of lime amendments.
Effects on other pollutants in runoff
Studies indicate spent lime is effective at removing metals, but there is limited information on effects of lime on other pollutants in stormwater runoff.
- Shrestha et al. (2019) found that spent lime in leachate from bioretention plots did not affect heavy metal concentrations of Cu and Mn, reduced Al, Fe and Zn and slightly increased Ni (0.016 in spent lime vs. 0.013 mg L−1 in non-spent lime plots). Heavy metal concentrations of Cd, Cr and Pb in all treatments were <0.001 mg L−1.
- Barr Engineering (2014) conducted a field study to determine the retention of phosphorus and metals in a permeable reactive barrier containing spent lime. They observed removal rates of 57, 57, 60, 86, 66, and 31 percent for TSS, aluminum, iron, lead, zinc, and copper respectively.
- DeBusk et al. (1997) found that use of limerock in a sand filter treating spiked stormwater runoff reduced concentrations of Cu, Cd, and Ni by 31, 81, and 31 percent, respectively.
- Adhikari et al. (2016) found spent lime reduced Cd and Pb concentrations to below detection (2 and 20 ug/L, respectively) in a column study with spiked stormwater runoff.
- Elliott et al. (1990) conducted laboratory studies to determine water soluble fractions of metals in Al- and Fe- water treatment residuals. They found water-soluble fractions of 5.8, 1.0, 1.0, 0.6, 4.2, and 0.5% for Cd, Cu, Cr, Ni, Pb, and Zn, respectively, indicating these metals are relatively strongly sorbed to lime.
- Roychowdhury et al. (2015) conducted laboratory batch sorption studies to determine removal of acidity and metals from acid mine drainage-impacted water using calciumwater treatment residuals. The filter media removed more than 99% of the initial Fe, Al, Zn, Pb, As, Mn, and 44% of the initial SO4-2. pH increased from 2.27 to 7.8. Desorption experiments showed that the metals were irreversibly bound to the WTRs and were not released back to the water.
- Shrestha et al. (2019) observed reductions in NH4+ concentrations were also observed due to spent lime but with variable significance across the different compost levels, whereas NO3− concentrations were higher in plots with spent lime than plots without spent lime.
Lime is effective at reducing turbidity, and coagulation and precipitation reactions are likely to reduce bacteria numbers, particularly at elevated pH (Grabow et al., 1978; Bennett, 2003).
There are few studies on the retention of organic chemicals by spent lime. Medina et al. (2007) observed moderate retention (41%) of PAHs in a contaminated soil in Ohio.
Effects on plant growth and microbial function
| Lime improves plant response in acidic soils, primarily by increasing nutrient availability and improving microbial function. In other soils, lime may increase pH and reduce availability of phosphorus. |
Lime is widely used in agricultural and other soils if the soil pH is too low. Lime is typically applied as a fine powder, although other forms are also applied, including burnt lime (quick lime), caustic lime that is predominantly the chemical calcium oxide, CaO, and hydrated lime, often referred to synonymously as slaked lime that is predominantly the chemical calcium hydroxide, Ca(OH)2. Added in proper amounts, lime can enhance uptake of nutrients, improve soil structure and hydraulic properties, and reduce potential toxicity of some metals, all beneficial to vegetation. Potential drawbacks include the caustic nature of some lime products, slow action to raise pH, and negative effects on soil hydraulic properties if too much lime is utilized ([12]; Pabian et al., (2012); Goulding, 2016).
Wandruszka (2006) demonstrated that excess lime in soil can inhibit phosphorus uptake by plants, but this is unlikely to be a concern in most stormwater applications due to relatively high concentrations of phosphorus in stormwater runoff. Afif et al. (1995) observed mixed results of applying lime to soil, with lime being ineffective in soils high in aluminum or iron due to phosphorus limitations to plants.
Fuentes et al. (2006) demonstrated that below-surface lime placement in acidic soils is effective for correcting soil acidity and that microbial activity and nitrification can be enhanced. Badalucco et al. (1992) observed increased CO2 evolution and microbial biomass and a shift from fungi to bacteria in a lime-amended soil. Lupwayi et al. (2020) observed a similar shift toward bacteria-dominated soils with lime application. Mühlbachová and Tlustoš (2006), studying the effects of liming on a mined site, observed soil liming increased microbial biomass, but the level of species diversity remain unchanged even though the microbial compositions of the damaged and restored sites were different.
Lime application to acidic soils can be expected to improve vegetative growth and microbial function if the soil pH is kept below about 7.5.
Standards and classification
No standards or classification were found in the literature.
Distributors
Below is a list of facilities in Minnesota that use lime in public water supplies for softening and generate spent lime.
- Bloomington
- Breckenridge
- Eden Prairie
- Fergus Falls
- Gilbert
- Little Falls
- Mankato
- Marshall
- Minneapolis
- Saint Cloud
- Saint Paul Regional Water Services
- Staples
- Thief River Falls
- Tonka Bay
- White Bear Lake
- East Grand Forks
- Fairmont
- Granite Falls
- Moorhead
- Richfield
- Morris
- Pipestone
Test methods
Standard test methods for spent lime were not found in the literature. Test methods are described in several reports (Shrestha et al., 2019; Elliott et al., 1990; Elliott et al., 2002; Baker et al., 2005).
Aging
Spent lime should not undergo structural or chemical changes over time, though there is insufficient data in the literature on this topic. The phosphorus binding capacity of media amended with spent lime can be calculated knowing the concentrations of calcium (Brock et al., 2007). Adhikari calculated a sorption capacity of 44 mg-P/g-lime.
Storage, handling, and field application
Storage
Keep quick lime dry until used. Normal temperatures and pressures do not affect the material.
Handling
Lime materials can be corrosive and cause irritation when handled. Material safety data sheets (MSDS) for some lime compounds are provided below.
- Calcium hydroxide
- Agricultural spent lime from sugar beet manufacturing
- Quicklime
- Hydrated lime
- Calcium oxide
Handling guidance includes the following:
- Not a hazardous waste either by listing or characteristic
- Corrosive material; avoid any release of dust during transportation, by using tight tanks for powders and covered trucks for pebbles.
- Following neutralization either at spill sites, the resultant sludge can be disposed of to a secure landfill. Or consult with environmental regulatory agencies for guidance on acceptable disposal practices.
- Exposure to quick lime may cause irritation or caustic burns to the moist mucous membranes of the nose, throat, and upper respiratory system. Exposure of sufficient duration to wet quick lime can cause serious, potentially irreversible tissue (skin or eye) destruction in the form of chemical (caustic) burns. Promptly remove dusty clothing or clothing which is wet with cement fluids and launder before reuse. Wash thoroughly after exposure to dust or quick lime mixtures or fluids. Where prolonged exposure to quick lime products might occur, wear impervious clothing and gloves to eliminate skin contact. Wear sturdy boots that are impervious to water to eliminate foot and ankle exposure. Avoid actions that cause dust to become airborne. Use local or general exhaust ventilation to control exposures below applicable exposure limits. Use NIOSH/MSHA approved (under 30 CFR 11) or NIOSH approved (under 42 CFR 84) respirators in poorly ventilated areas, if an applicable exposure limit is exceeded, or when dust causes discomfort or irritation. (Advisory: Respirators and filters purchased after June 10, 1998 must be certified under 42 CFR 84.) Ventilation Use local exhaust or general dilution ventilation to control exposure within applicable limits.
- Eye Protection: Where potentially subject to splashes or puffs of quick lime, wear safety glasses with side shields or goggles. In extremely dusty environments and unpredictable environments wear unvented or indirectly vented goggles to avoid eye irritation or injury. Contact lenses should not be worn when working with quick lime.
Application
There are few studies that provide design information for spent lime in stormwater applications. There are numerous studies in the literature describing use of lime for agricultural and other soil applications. Lime is primarily used to adjust soil pH in acidic soils, thereby improving uptake of specific elements and reducing potential toxicity associated with other elements, particularly metals. Since phosphorus can be limiting in soils, these studies typically have the goal of increasing pH to neutral values. For stormwater applications, phosphorus will not be limiting because of constant inputs of phosphorus from stormwater runoff. Raising the pH is therefore less of a concern except for the potential to impact vegetation. An appropriate plant assemblage should therefore be selected based on the final pH in the amended media. For more information on pH ranges for individual plant species, see Plants for Stormwater Design (Shaw and Schmidt, 2003; pH ranges are given in the descriptions for each species).
The primary concern with lime additions is on reduced hydraulic conductivity associated with excess lime application. In a study by Barr Engineering (2014), annual maintenance consisted of removal of accumulated material on the top of the cell and mixing the top 1-foot of the lime material with a shovel. They recommended future designs consider the use of a pond or plunge pool to settle out sand, leaves, and sticks to minimize the accumulation of material on the surface of the spent lime and also reduce the frequency of maintenance.
Sustainability
Since spent lime is a byproduct produced from water softening, it is a sustainable product for stormwater applications. Salih et al. (2019) estimated water utilities across the United States are currently generating approximately 3.2 million tons of lime sludge per year. Several cities in Minnesota soften their municipal water with lime, and the number of facilities using lime is increasing as a means of reducing demand for water softening in individual homes.
References
- Adhikari, R.A., KC Bal Krishna, and R. Sarukkalige. 2016. Evaluation of phosphorus adsorption capacity of various filter materials from aqueous solution. Adsorption Science & Technology. Vol. 34(4–5):320–330
- Afif, E., A. Matar, and J. Torrent. 1993. Availability of Phosphate Applied to Calcareous Soils of West Asia and North Africa. Soil Science Society of America Journal. Volume 57, Issue3. Pages 756-760
- Agyin-Birikorang, S. G.A. O’Connor, and T.A. Obreza. 2009. Drinking Water Treatment Residuals to Control Phosphorus in Soils. U.S. Department of Agriculture, UF/IFAS Extension Service, University of Florida, IFAS, Florida A & M University Cooperative Extension Program, and Boards of County Commissioners.
- Badalucco, L., S. Grego, S. Dell'Orco and P. Nannipieri. 1992. Effect of liming on some chemical, biochemical, and microbiological properties of acid soils under spruce (Picea abies L.). Biology and Fertility of Soils volume 14, pages 76–83.
- Bain, J., D. Blowes, D. Smyth, C. Ptacek, J. Wilkins, and R. Ludwigh. 2006. Permeable Reactive Barriers for in-situ Treatment of Arsenic-contaminated Groundwater. In Proceedings, Fifth International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, May 22 - 25, 2006. Battelle Press, Columbus, OH, H-38, ISBN1574771574, (2006).
- Baker, J., H. van Leeuwen, and D.J. White. 2005. Applications for Reuse of Lime Sludge from Water Softening. Iowa State University.
- Baker, M.J., D.W. Blowes. 1997. Phosphorous adsorption and precipitation in a permeable reactive wall: Applications for wastewater disposal systems. Waterloo Centre for Groundwater Research, Ontario, C.J. Placek, and Univ. of Waterloo, Ontario.
- Bennett, D.D., S.E. Higgins, R.W. Moore, and R.B. Aguedo. 2003. Effects of Lime on Salmonella enteritidis Survival In Vitro. The Journal of Applied Poultry Research 12(1). DOI: 10.1093/japr/12.1.65.
- Bhasker, P., B. Boluk, A. Banerjee, A. Shafikhani. 2019. Effect of Lime Stabilization on the Unsaturated Hydraulic Conductivity of Clayey Soil in Texas. Eighth International Conference on Case Histories in Geotechnical Engineering.
- Birdsey Jr., P.W. 1985. Coprecipitation of Phosphorus With Calcium Carbonate in Bear Lake, Utah – Idaho. M.S. Thesis. Utah State University.
- Boardman, D.I., S. Glendinning, and C.D.F. Rogers. 2001. Development of stabilisation and solidification in lime–clay mixes. Geotechnique. Volume 51 Issue 6, pp. 533-543.
- Brock, E.H., Q. M. Ketterings, P. Kleinman. 2007. Measuring and Predicting the Phosphorus Sorption Capacity of Manure-Amended Soils. Soil Science 172(4):266-278. DOI: 10.1097/ss.0b013e318032ab2e
- Bronstein, K. 2005. Permeable Reactive Barriers for Inorganic and Radionuclide Contamination. U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response Office of Superfund Remediation and Technology Innovation Washington, DC.
- Choquette, M., A. Bérubé, J. Locat. 1987. Mineralogical and microtextural changes associated with lime stabilization of marine clays from eastern Canada. Applied Clay Science. Volume 2, Issue 3, pages 215-232.
- Clark, J.S., and R.C. Turner. 1955. Reactions between Solid Calcium Carbonate and Orthophosphate Solutions. Can. J. Chem. Downloaded from www.nrcresearchpress.com.
- DeBusk, T.A., and M.A. Langston, B.R. Schwegler, S. Davidson. 1997. An Evaluation of Filter Media for Treating Stormwater Runoff. Fifth Biennial Stormwater Research Conference November 5-7,1997.
- Di Santi, M., B.D. Buò, E. Fratalocchi, and T. Länsivaara. 2020. Lime Treatment of a Soft Sensitive Clay: A Sustainable Reuse Option. Geoscience. 10(5):182. https://doi.org/10.3390/geosciences10050182
- Dunets, C.S., Y. Zheng, and M. Dixon. 2015. Use of phosphorus-sorbing materials to remove phosphate from greenhouse wastewater. Environ Technol. 36(13-16):1759-70. doi: 10.1080/09593330.2015.1009497.
- Elkady, T.Y., A. Shaker, and M.Al-Shamrani. 2016. Hydraulic Conductivity of Compacted Lime-Treated Expansive Soils. Fourth Geo-China International Conference. https://doi.org/10.1061/9780784480014.007.
- Elliott, H.A., B.A. Dempsey, and P.J. Maille. 1990. Content and Fractionation of Heavy Metals in Water Treatment Sludges. J. Environ. Qual. 19:330-334.
- Elliott, H.A., G.A. O’Connor, P. Lu, and S. Brinton. 2002. Influence of Water Treatment Residuals on Phosphorus Solubility and Leaching. J. Environ. Qual. 31:1362–1369.
- Emery, K.M. 2013. New Morbid Terminology: Quicklime.
- Erickson, AJ, Kozarek, JL, Kramarczuk, KA, and Lewis, L. 2020. Biofiltration Media Optimization – Phase 1 Final Report. Project Report No. 593, St. Anthony Falls Laboratory, University of Minnesota, Minneapolis, MN. December 2020.
- Erickson, A.J. 2005. Enhanced Sand Filtration for Storm Water Phosphorus Removal. M.S. Thesis. University of Minnesota.
- Fuentes, J.P., D.F. Bezdicek M. Flury, S. Albrecht, and J.L. Smith. 2006. Microbial activity affected by lime in a long-term no-till soil. Soil & Tillage Research 88:123–131.
- Gao, Y.Gao, H. Qian, X. Li, J. Chen and H. Jia. Effects of lime treatment on the hydraulic conductivity and microstructure of loess. Environmental Earth Sciences volume 77, Article number: 529.
- Goulding, K.W.T. 2016. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016 Sep; 32(3): 390–399. doi: 10.1111/sum.12270.
- Grabow, W.O., I.G Middendorff, and N.C Basson. 1978. Role of lime treatment in the removal of bacteria, enteric viruses, and coliphages in a wastewater reclamation plant. Appl Environ Microbiol. 35(4):663-9. doi: 10.1128/AEM.35.4.663-669.1978.
- Hunter, D. 1988. Lime‐Induced Heave in Sulfate‐Bearing Clay Soils. Journal of Geotechnical Engineering Vol. 114, Issue 2. https://doi.org/10.1061/(ASCE)0733-9410(1988)114:2(150)
- Lang, J. 2009. Experimental Study on the Use of Lime Sludge for Construction: An Example for Sustainability. Case Western Reserve University, Cleveland, OH.
- Locat, J., H. Trembaly, and S. Leroueil. 1996. Mechanical and hydraulic behaviour of a soft inorganic clay treated with lime. Canadian Geotechnical Journal. DOI:10.1139/T96-090-311. 33:654-669.
- Ludwig, R.D., D.J.A. Smyth, D.W. Blowes, L.E. Spink, R.T. Wilkin, D.G. Jewett, and C.J. Weisener. 2009. Treatment of Arsenic, Heavy Metals, and Acidity Using a Mixed ZVI-Compost PRB. Environ. Sci. Technol. 43:6:1970–1976. https://doi.org/10.1021/es802394p.
- Lupwayi, N.Z., M.A. Arshad, R.H. Azooz, and Y.K. Soon. 2009. Soil microbial response to wood ash or lime applied to annual crops and perennial grass in an acid soil of northwestern Alberta. Canadian Journal of Soil Science 89(2):169-177. DOI:10.4141/CJSS08007
- Medina, V.F., S.A. Waisner, A.B. Morrow, C.C. Nestler, and M. Jones. 2007. Evaluation of Lime and Persulfate Treatment for Mixed Contaminant Soil from Plum Brook Ordnance Works (Sandusky, OH). US Army Corps of Engineers. Engineer Research and Development Center. ERDC/EL TR-07-19.
- Mühlbachová, G., and P. Tlustoš. 2006. Effects of liming on the microbial biomass and its activities in soils long-term contaminated by toxic elements. PLANT SOIL ENVIRON., 52,(8): 345–352
- Nguyen, T.T.H., Y.J. Cui, G. Herrier, A.M. Tang, M.G. Winter, D.M. Smith, P.J.L. Eldred, D.G. Toll. 2015. Effect of lime treatment on the hydraulic conductivity of a silty soil. Geotechnical Engineering for Infrastructure and Development.
- Pabian, S.E., N.M. Ermer, W.M. Tzilkowski, and M.C. Brittingham. 2012. Effects of Liming on Forage Availability and Nutrient Content in a Forest Impacted by Acid Rain. PLoS One. 2012; 7(6): e39755. doi: 10.1371/journal.pone.0039755
- Ranjbar, E., R. Ghiassi, and Z. Akbary. 2017. Lead removal from groundwater by granular mixtures of pumice, perlite and lime using permeable reactive barriers. Water and Environment Journal 31(1):39–46. DOI: 10.1111/wej.12223
- Rogers, C.D.F. and S. Glendinning. 2000. Lime Requirement for Stabilization. Transportation Research Record. 1721(1):9-18. doi:10.3141/1721-02https://doi.org/10.3141/1721-02.
- Roychowdhury, A., D. Sarkar, R. Datta. 2019. Removal of Acidity and Metals from Acid Mine Drainage-Impacted Water using Industrial Byproducts. Environmental Management 63. DOI: 10.1007/s00267-018-1112-8.
- Salih, H.H., C.L. Patterson, J.Li, J. Mock. 2019. Utilization of Water Utility Lime Sludge for Flue Gas Desulfurization in Coal-Fired Power Plants: Part I. Supply–Demand Evaluation and Life Cycle Assessment. Energy & Fuels 32(6). DOI: 10.1021/acs.energyfuels.8b00823
- Sherwood, P. 1993. Soil Stabilization with Cement and Lime. Transport Research Laboratory. ISBN: 9780115511714.
- Shrestha, P., M.T. Salzl, I.J. Jimenez, N. Pradhan, M. Hay, H.R. Wallace, J.N. Abrahamson, and G.E. Small. 2019. Efficacy of Spent Lime as a Soil Amendment for Nutrient Retention in Bioretention Green Stormwater Infrastructure. Water. 11(8):1575. https://doi.org/10.3390/w11081575.
- Trana, T.D., Yu-Jun Cui, A. M. Tang, M. Audiguier, R. Cojeana. 2014. Effects of lime treatment on the microstructure and hydraulic conductivity of Héricourt clay. Journal of Rock Mechanics and Geotechnical Engineering. Volume 6, Issue 5, Pages 399-404.
- Turner, T., R. Wheeler, A. Stone, and I. Oliver. 2019. Potential Alternative Reuse Pathways for Water Treatment Residuals: Remaining Barriers and Questions—a Review. Water, Air, & Soil Pollution volume 230, Article number: 227.
- U.S EPA. 2011. Drinking Water Treatment Plant Residuals Management Technical Report Summary of Residuals Generation, Treatment, and Disposal at Large Community Water Systems. EPA 820-R-11-003.
- von Wandruszka, R. 2006. Phosphorus retention in calcareous soils and the effect of organic matter on its mobility. Geochem Trans. 7:6. doi: 10.1186/1467-4866-7-6.
- Wang, C., L. Bai, Y. Pei, and L. A. Wendling. 2014. Comparison of metals extractability from Al/Fe-based drinking water treatment residuals. Environmental Science and Pollution Research. Volume 21:13528–13538.
- Wild, S., M.R. Abdi, and G. Leng-Ward. 2018. Sulphate Expansion of Lime-Stabilized Kaolinite: II. Reaction Products and Expansion. Clay Minerals. 28(4): 569-583. doi:10.1180/claymin.1993.028.4.07.
- Yanamadala, V.. 2005. Calcium Carbonate Phosphate Binding Ion Exchange Filtration and Accelerated Denitrification Improve Public Health Standards and Combat Eutrophication in Aquatic Ecosystems. Water Environ Res. 77(7):3003–3012.
This page was last edited on 21 February 2023, at 21:08.