Difference between revisions of "Soil chemical properties and processes"
m |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | |||
| − | |||
| − | |||
[[File:Technical information page image.png|100px|left|alt=image]] | [[File:Technical information page image.png|100px|left|alt=image]] | ||
| Line 472: | Line 469: | ||
*[https://www.iuss.org/19th%20WCSS/Symposium/pdf/1216.pdf Characterisation of organic phosphorus compounds in soil by phosphatase hydrolysis] | *[https://www.iuss.org/19th%20WCSS/Symposium/pdf/1216.pdf Characterisation of organic phosphorus compounds in soil by phosphatase hydrolysis] | ||
*[https://www.scielo.br/j/sa/a/Sqj6t9gP57mFRq4NqsYcMtS/?lang=en&format=pdf Exchangeable Aluminum Evaluation in Acid Soils] | *[https://www.scielo.br/j/sa/a/Sqj6t9gP57mFRq4NqsYcMtS/?lang=en&format=pdf Exchangeable Aluminum Evaluation in Acid Soils] | ||
| + | |||
| + | [[Category:Level 2 - Technical and specific topic information/soils and media]] | ||
Latest revision as of 01:22, 1 December 2022
| Overview of soil chemical properties and associated activities affecting soil chemical properties and processes. Click on links to go to a specific section. | |||
| Property | Effects | Desired value1 | Management strategies |
| Phosphorus | An essential nutrient for plants and animals | Optimum concentration is 30 to 50 mg-P/kg-soil (ppm) | Add organic or inorganic fertilizer when deficient; maintain soil pH of 6-8; utilize appropriate vegetation; add amendment to retain P if leaching is a concern |
| Nitrogen | An essential nutrient for plants and animals | Optimum concentration is 25-50 mg-N/kg-soil | Add organic or inorganic fertilizer when deficient; keep soil aerated; promote denitrification if leaching is a concern |
| Major cations | Calcium (Ca), magnesium (Mg), potassium (K) are essential nutrients for plants and animals; these cations may affect soil properties (e.g. crusting with excess sodium) or soil processes (e.g. buffering) | Ca: > 300 mg/kg-soil; Mg: > 35 mg/kg-soil; K: 40-80 mg-kg-soil; sodium: less than 15% of soil cation exchange capacity | Lime to increase Ca and Mg; add K fertilizer if deficient; irrigate soil thoroughly if sodium levels are high |
| Sulfur | An essential nutrient for plants and animals | Optimum concentration is 30-40 mg-S/kg-soil | Amend soils with sulfur product if deficient (be aware of pH effects) |
| Trace metals | Some trace metals and metalloids are essential for plants and animals (molybdenum (Mo), copper (Cu), boron (B), manganese (Mn), iron (Fe), zinc (Zn)); most trace metals are toxic above certain concentrations | Mo: 2 mg/kg-soil; B: 0.5-4 mg/kg-soil; Cu: 2-50 mg/kg-soil; Zn: 1-200 mg/kg-soil; Mn: 2-25 mg/kg-soil; Fe: 20-30 mg/kg-soil. For non-essential trace metals, toxic effects vary with vegetation. | Adjust pH as appropriate; add organic matter to sorb contaminants; fertilize if deficient |
| Cation exchange capacity | Affects the availability of nutrients and pollutants | 12-40 meq/100 g-soil | Amend soil with organic matter |
| Electrical conductivity | An indicator of salt concentration in soil. Salts affect availability and transport of nutrients and pollutants. | Less than 8 dS/cm is desirable | Irrigate soil to flush salts; add well-composted organic matter |
| Organic matter (carbon) | Many important effects in soil, including providing nutrients, improving soil physical properties (e.g. structure, porosity, infiltration), retaining pollutants, and stabilizing soil pH and buffering capacity | 2-8 % of soil, by weight | Add composted organic matter. The organic matter source varies with the objective. Manures and biosolids have higher percentages of available nutrients, wood-based sources will retain nitrogen, and yard waste sources are intermediate between these. |
| Base saturation | An indicator of the extent to which cation exchange capacity is attributable to calcium, magnesium, and potassium. Low base saturation may reflect soils high in exchangeable aluminum, which can be detrimental to plants | 40-80% for calcium, 10-40% for magnesium (Mg), and 1-5% for potassium (K) | Liming will increase base saturation |
| Enzymes | Enzymes mediate many chemical processes in soil | Varies with specific enzyme and soil process being targeted | Add organic matter, rotate vegetation if applicable, use cover vegetation |
| Salinity | High salinity reflects soils with high sodium concentrations, which negatively impacts soil structure | Take electrical conductivity readings, with values greater than 16 dS/cm indicating sodic soils | Determine and reduce sources of sodium (e.g. road salt); irrigate to flush sodium |
| Sodium adsorption ratio | An indicator of sodium concentrations in soil. Elevated sodium concentrations negatively impacts soil structure | Values great than 13 indicate sodic soils | Determine and reduce sources of sodium (e.g. road salt); irrigate to flush sodium |
| pH | Affects many soil processes, such as microbial activity, affects availability and mobility of nutrients and pollutants | 6-8 | Lime if pH is low; add sulfur if pH is high; organic matter can buffer pH, depending on the organic matter source |
| 1Values vary with soil, vegetation, and management goals. Recommended sources for more information: [1], [2], [3], [4], [5] | |||
Soil chemical properties include concentrations of specific chemicals (e.g. phosphorus, nitrogen, carbon, major cations (calcium, magnesium, sodium, potassium), sulfur, trace metals and elements), pH, cation exchange capacity cation exchange capacity, base saturation, salinity, sodium adsorption ratio, enzymes, and electrical conductivity. These properties affect processes such as nutrient cycling, biologic activity, soil formation, pollutant fate, and erosion.
This page provides an overview of soil chemical properties, processes they affect, effects of human activities, discussion of stormwater applications, and links to related topics, including information on sampling, testing, and soil health assessments.
Soil chemical properties
Soil chemical properties discussed below include phosphorus, nitrogen, major cations, trace metals, cation exchange capacity, electrical conductivity, enzymes, organic matter and carbon, base saturation, salinity, sodium adsorption ration, and pH.
Phosphorus
Phosphorus constitutes about 0.2 percent of a plant’s dry weight, where it is primarily a component of tissue molecules such as nucleic acids, phospholipids, and adenosine triphosphate (ATP). Along with nitrogen, phosphorus is often a limiting nutrient in soil. Soils limited in phosphorus reduce plant growth and development, while excess phosphorus can be exported from soil and enter freshwater bodies.
Approximately 30 to 65 percent of total soil phosphorus is in organic forms and the remaining 35 to 70 percent in inorganic forms. Soil microorganisms play a key role in processing and transforming organic forms into plant available forms. Inorganic phosphorus forms include the following:
- plant-available phosphorus, comprised of inorganic phosphorus dissolved in soil water;
- phosphorus attached (sorbed) to clay surfaces, iron (Fe), aluminum (Al), and calcium (Ca) oxides in soil, which can be released slowly for plant uptake; and
- mineral phosphorus (e.g. apatite), which is very slowly released.
Phosphorus is typically measured in a laboratory with one of the following methods.
- Bray Method: use on acidic and neutral soils
- Olsen Method: preferred on high pH soils
- Mehlich-3 Method: provides a better indicator of plant-available phosphorus
Phosphorus can also be determined in the field with appropriate equipment or with test strips. Test strips are less accurate but may be suitable for identifying phosphorus deficiencies.
The soil phosphorus cycle is somewhat complicated since phosphorus is affected by soil mineralogy and chemistry and by soil biotic processes.
- Organic matter: Phosphorus availability generally increases with increasing soil organic matter since phosphorus is released through mineralization of organic matter. Fresh (non-composted) organic sources have greater amounts of available phosphorus.
- Clay: Soils with high clay content have high phosphorus retention capacity.
- Soil mineralogy: Soils with high concentrations of aluminum, iron, and calcium have high phosphorus retention capacity.
- Soil pH: Optimum soil pH between 6 and 7 will result in maximum phosphorus availability. At low pH (acidic soils), soils have greater amounts of aluminum and iron, which form very strong bonds with phosphate. At high pH phosphate tends to precipitate with calcium.
- Temperature, moisture, and soil aeration can affect the rate of phosphorus mineralization from organic matter decomposition.
Stormwater application: Stormwater runoff typically contains about 0.2-0.6 mg/L of total phosphorus, though this varies with land use and season. Particulate phosphorus accounts for about 50-75% of total phosphorus, being higher in areas lacking tree canopy cover. Dissolved forms are approximately five times more bioavailable than particulate forms. Phosphorus is not limiting in most stormwater applications. In many applications where engineered media are employed, phosphorus represents a risk for surface receiving waters. Link here for more information.
Additional reading
- Phosphorus in stormwater
- Phosphorus Basics: Understanding Phosphorus Forms and Their Cycling in the Soil
- Understanding phosphorus in Minnesota soils
- Soil phosphorus - USDA-NRCS
- Phosphorus
- How to Effectively Manage Phosphorus Levels in the Soil
Nitrogen
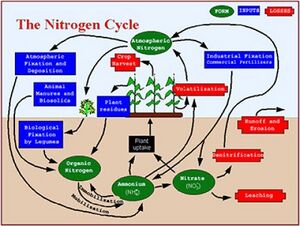
Nitrogen constitutes about 1 percent of a plant’s dry weight, where it plays a role in the growth of tissues and cells found within the plant, as well as the formation of chlorophyll. Along with phosphorus, nitrogen is often a limiting nutrient in soil. Soils limited in nitrogen reduce plant growth and development, while excess nitrogen can be exported from soil and enter freshwater bodies, including groundwater.
Nitrogen occurs in both organic and inorganic forms in soil, with ammonium (NH4+) and nitrate (NO3-) being the dominant inorganic forms.
Measurement of nitrogen in soils depends on the form being measured.
- Soil Nitrate And Extractable Ammonium By Flow Injection Analyzer Method (cadmium reduction method)
- Total Kjeldahl nitrogen (wet oxidation method)
- Total Nitrogen And Carbon - Combustion Method (Dumas Method)
The nitrogen cycle in soil is somewhat complex, with the following important processes (Plant and Soil Sciences e-library).
- Fixation is the process of converting dinitrogen gas (N2) to chemically reactive forms. Bacteria can convert nitrogen to organic forms through fixation. Fixation can occur either in free-living organisms or symbiotically in association with legumes.
- Once nitrogen is fixed, microorganisms can convert organic forms to inorganic forms through mineralization. This often is a two-step process where proteins are converted to simple compounds and then to ammonium.
- Nitrification is a two-step process in which microbes convert ammonium to nitrite and then nitrate. Nitrite is toxic to plants, but it is typically rapidly converted to nitrate, which is a plant available form.
- Denitrification is a process in which microbes convert nitrate to nitrogen gas. This occurs in the absence of oxygen.
- Volatilization involves the conversion of urea to ammonia, which is lost as a gas. This typically occurs under moist and warm conditions.
- Nitrate not taken up by plants is readily leached in aerated soils, eventually reaching groundwater, surface waters through baseflow, or being denitrified in the absence of oxygen.
- Nitrogen can temporarily become unavailable through conversion to organic forms or assimilation in plant or animal tissue.
Stormwater application: Stormwater runoff typically contains about 2-3 mg/L of total nitrogen, with roughly equal proportions of nitrate and reduced forms (ammonia and organic nitrogen). Nitrogen is not limiting in most stormwater applications and represents a relatively low risk for receiving waters in most situations. Link here for more information.
Additional reading
- Plant and Soil Sciences e-library - Forms of Nitrogen in the Soil
- Understanding Nitrogen in Soils - University of Minnesota Extension
- Soil nitrogen - USDA-NRCS
- What Happens to Nitrogen in Soils?
- Nitrogen Basics – The Nitrogen Cycle - Cornell University
Major cations
Cations are positively charged elements. Major cations in soil include calcium (Ca2+), magnesium (Mg2+), sodium (Na+), and potassium (K+). These elements are utilized in smaller quantities than phosphorus or nitrogen, but calcium, magnesium, and potassium are essential plant nutrients since they are involved in a variety of plant functions and metabolic processes, while all four can have adverse effects on soil or plants.
These elements can be determined as part of a metal scan using Inductively Coupled Plasma Emission Spectrometry (ICP-AES), but less expensive methods are suitable if sampling is just for these major cations. These methods include ion chromatography, atomic absorption spectroscopy, and flame photometry.
While these cations are generally not limiting in soil and they rarely occur at toxic levels, they can compete with each other and affect the fate of other elements in soil. Examples include but are not limited to the following.
- Excess calcium may affect the availability of phosphorus, potassium, magnesium, boron, copper, iron, or zinc.
- Excess sodium can be toxic to plants and disperse clays, resulting in reduced infiltration and soil surface crusting.
- Excess potassium can affect plant uptake of nutrients.
- Excess magnesium can form soil crusting and negatively affect soil structure.
These elements are often considered in the context of soil cation exchange capacity (CEC), an important property affecting the fate of other chemicals in soil, including pollutants. For information on soil CEC, link here.
Stormwater application: These elements are generally not a concern in stormwater applications and represent a low risk to receiving waters. An exception is sodium in stormwater management systems affected by road salt applications.
Additional reading
- Calcium in plants and soil
- Magnesium in plants and soil
- Potassium in plants and soil
- Calcium in the soil
- Sodium affected soils
- Soil potassium
- High magnesium soils
Sulfur
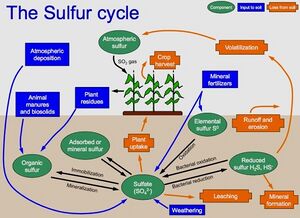
Sulfur is an essential nutrient and plays a major role in the formation of proteins. It is taken up by plants in similar amounts as phosphorus. More then 90 percent of the sulfur in soil occurs in organic forms. Soil microbes mineralize this sulfur, converting it to sulfate, the form taken up by plants. Other sources of sulfur include minerals, atmospheric deposition, and in some cases fertilizer.
Sulfur concentrations in soil are determined in the laboratory. Several reliable methods exist, such as high-performance liquid chromatography and inductively coupled plasma atomic emission spectroscopy. Analysis methods, including digestion procedures, depend on what is being tested (e.g. elemental sulfur, organic sulfur, sulfate).
Stormwater application: Sulfur is not a concern in most stormwater applications. Sulfur deficiencies may occur in well-drained sandy soils.
Additional reading
- Technical Bulletin: Sulfur in Soils
- Sulfur in Plants and Soil - the 4th Macronutrient
- Sulfur Gardening Usage: Importance Of Sulfur In Plants
- Sulfur
- Sulfur for Minnesota soils
- The Importance of Sulfur in Your Soil
Trace metals
Trace metals include cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), manganese (Mn), mercury (Hg), molybdenum (Mo), nickel (Ni), selenium (Se), and zinc (Zn). Arsenic (As), boron, and selenium are metalloids but are usually included in discussions of metals in soil. Heavy metals refers to metals with a density of more than 7 g/cm3 (Pb, Cd, Ni, Hg, Cr). Minerals (rocks) are the primary source of most trace metals in soil.
Some trace metals are essential micronutrients (e.g. boron, zinc, manganese, copper, molybdenum). In higher concentrations, trace metals are environmental pollutants and may be toxic to plants and soil biota.
Soil samples are typically collected for total metals, meaning samples are not filtered. For dissolved metal concentrations, samples are filtered using a 0.45 micron filter. From an environmental perspective, dissolved metal concentrations more accurately reflect potential risk to receptors, since most metal bound to particles is retained in stormwater best management practices (bmps). Lab methods include the following.
- Inductively Coupled Plasma Emission Spectrometry (ICP-AES) - recommended due to lower detection limits
- atomic absorption spectroscopy
The behavior of trace metals in soil varies widely with soil properties. Processes affecting metal behavior include dissolution/solubilization, precipitation, hydrolysis/pH, exchange/adsorption, oxidation-reduction (redox), and chelation. Below is a brief summary of primary factors affecting trace metals in soil.
- Arsenic: iron (Fe) and aluminum (Al) contents, pH, redox potential, and competing anions (e.g. phosphorus)([6])
- Boron: pH, organic matter, aluminum and iron ([7])
- Cadmium: iron and manganese oxides, pH ([8])
- Chromium: Iron and aluminum oxides, pH ([9])
- Copper: organic matter ([10])
- Lead: pH and complexation with organic matter ([11])
- Manganese: redox, pH, organic matter ([12])
- Mercury: pH and redox potential ([13])
- Molybdenum: pH, organic matter ([14])
- Nickel: iron and manganese oxides, organic matter ([15])
- Selenium: iron oxides, pH, redox ([16])
- Zinc: iron and manganese oxides, pH ([17])
Stormwater application: Trace metals in stormwater runoff may exceed aquatic life standards for receiving waters, particularly in heavy transportation areas, industrial areas, and areas affected by contaminated soils. Metals of greatest concern include lead, arsenic, cadmium, and copper. Except for sandy soils with limited organic matter, most soils and engineered stormwater media effectively retain metals. There have been concerns about buildup of metals in soil and stormwater media receiving high inputs from runoff.
Additional reading
- An Overview of Soil Trace Metal Chemistry
- Behavior of Metals in Soils
- Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals
- Essential Elements for Plant Growth - Macronutrients and Micronutrients
- Soil micronutrients - Clemson University
Cation exchange capacity
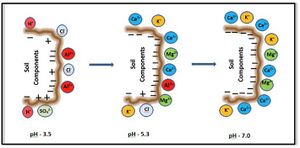
Cation exchange capacity (CEC) is the total capacity of a soil to hold exchangeable cations. It affects the fate of other soil chemicals, including nutrients and pollutants, and provides a buffer against soil acidification. Clay soils have a higher CEC than sandy soils, but soil organic matter has the greatest effect on a soil's CEC. The main ions associated with CEC are calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+). In acidic soils, aluminum (Al3+) and manganese (Mn2+) are important.
Units for CEC are millequivalents per mass of soil (meq/100-g soil). Typical values range from less than 5 meq/100-g soil for sands to more than 30 for clays. CEC varies with soil pH, which affects the cations involved in reactions. Adding organic matter will increase a soil's CEC since organic matter has a CEC about 4 times greater than that of clay.
CEC and exchangeable ions are determined in the laboratory.
- Cation exchange capacity - multiple methods are available for cation exchange capacity. For more information read here.
- Barium chloride Compulsive Exchange Method - recommended but is time consuming and generates a hazardous waste
- Ammonium Acetate Method - acceptable if soil pH is near 7.0
- Agronomic Soil Tests - estimates CEC from test extractable Ca, K, and Mg and some rapid measure of exchangeable acidity (see next bullet)
- Exchangeable Potassium, Calcium, Magnesium, Sodium And Estimated Cation Exchange Capacity
Stormwater application: CEC affects the availability of other chemicals and is particularly important in the availability of nutrients. Higher CEC represents more fertile soils and greater available nutrients for plant growth. In vegetated stormwater systems, particularly infiltration systems with sandy soils, CEC can be limiting. Addition of organic matter will increase soil CEC.
Additional reading
- Cations and Cation Exchange Capacity
- Fundamentals of Soil Cation Exchange Capacity (CEC)
- Soil-Nutrient Relationships
- Cation Exchange Capacity and Base Saturation
- Cation exchange capacity (CEC)
- Cation exchange capacity
Electrical conductivity
Electrical conductivity (EC) is the ability of a material to conduct (transmit) an electrical current. It is commonly expressed in units of milliSiemens per meter (mS/m) or deciSiemens per meter (dS/m). EC is primarily used to assess salt concentration in soil. Although EC does not provide a direct measurement of specific ions or salt compounds, it has been correlated to concentrations of nitrates, potassium, sodium, chloride, sulfate, and ammonia.
Values greater than 8 dS/m are considered to be moderately saline, while soils greater than 16 dS/m are considered strongly saline. Saline soils are most likely is soils impacted by a specific source, such as road salt, in arid climates, in irrigated soils, and in soils receiving large quantities of manure or biosolids.
EC can be measured in the field using a meter or electrodes. Meters should be properly calibrated.
Stormwater application: EC is not widely used in stormwater applications, but is a potentially useful indicator of soils impacted by road salts or other high salt materials.
Additional reading
- What Is Soil Electrical Conductivity? - Louisiana State University
- Soil Electrical Conductivity - USDA-NRCS Educator kit
- Soil Electrical Conductivity - USDA-NRCS Soil Health series
- Soil Electrical Conductivity - South Dakota Soil Health Coalition
- Soil electrical conductivity: a beginner’s guide to measurements
- Electrical Conductivity - What is Electrical Conductivity? - Soil Health Nexus
- Water and Soil Characterization - pH and Electrical Conductivity
Organic matter (carbon)

Soil organic matter affects chemical, physical, and biological properties and processes in soil. Chemically, soil organic matter affects the cation exchange capacity, provides nutrients, and affects the capacity for buffering changes in soil pH. These in turn affect the fate and transport of chemicals including nutrients and pollutants.
Recommended levels of organic matter are 2-8 percent by weight. Most Minnesota soils fall within this range. Engineered media mixes often have higher concentrations (15 percent or more).
The chemical composition and properties of organic matter vary with source and age. Sources such as bark, stems, and roots have low nutrient value compared to biosolids, manure, and fresh green organic materials. As organic materials age, nutrient levels decrease, particularly if the organic material is composted. Some sources, such as sphagnum peat, may lower soil pH.
Soil organic matter is measured in the field, usually with one of the following methods.
- organic carbon combustion
- organic matter Walkley-Black
- Organic matter loss on ignition - recommended due to ease of use and since organic matter is more commonly used compared to organic carbon
Stormwater application: Organic matter is an important component of engineered media in stormwater practices. It is the most important factor affecting retention of metals and most organic pollutants from stormwater runoff. Conversely, phosphorus and nitrate may be released from organic matter and therefore represent a potential concern for receiving waters. It is also an important source of nutrients.
Additional reading
- Soil organic matter - USDA-NRCS
- Soil organic matter - Cornell University
- Soil organic matter in cropping systems - University of Minnesota
- What Does Organic Matter Do In Soil? - Noble Research Institute
- Why Soil Organic Matter Is So Important - Sustainable Agriculture Research and Education
- Natural factors influencing the amount of organic matter - FAO
- What is soil organic carbon?
Base saturation
Base saturation is calculated as the percentage of CEC occupied by base cations (calcium, magnesium, potassium, sodium, hydrogen). As base saturation increases, pH increases. In soils where CEC is dominated by aluminum, base saturation is low and plant growth may be inhibited by the elevated aluminum concentration. Soils with high base saturation also have greater buffering capacity.
Base saturation does not provide an indication of exchangeable cations in soil. To determine these, cation exchange capacity and specific cation ratios must be determined. Normal ranges for exchangeable bases are 40-80% for calcium, 10-40% for magnesium (Mg), and 1-5% for potassium (K). The ratio of K to Mg should be between 0.2 to 0.3 for best uptake.
Stormwater application: Base saturation is not widely used in stormwater applications, but is recommended in acid soils where aluminum concentrations may be elevated. In soils amended with aluminum or iron products, base saturation may be also be recommended.
Additional reading
- Calculating Cation Exchange Capacity, Base Saturation, and Calcium Saturation - The Ohio State University
- Cation Exchange Capacity and Base Saturation - University of Georgia
- Base Saturation and Cation Exchange Capacity
- CROPTALK: BASE SATURATION: AN IMPORTANT SOIL FERTILITY CALCULATION
Enzymes
Enzymes in soil mediate numerous chemical reactions involved in soil nutrient cycling, transformation of plant and microbial debris, mineralization and transformation of organic matter within the carbon cycle, and transformation and degradation of potentially hazardous pollutants. The potential enzymes playing major roles in maintaining soil health are amylase, arylsulphatase, β-glucosidase, cellulase, chitinase, dehydrogenase, phosphatase, protease, and urease. For a discussion of the processes affected by specific enzymes, link here.
Several analysis methods exist, with no specific recommendation due to the variety of enzymes that can be tested. A description of analysis methods is found here.
Stormwater application: Enzymes are excellent indicators of soil health as changes of enzymes occur sooner than other parameters, thus providing early indications of changes in soil health. They provide measures of microbial activity, soil productivity, and inhibiting effects of pollutants. Thus, while not directly related to stormwater processes, they provide excellent indicators of how well a soil or media will function.
Additional reading
- Soil Enzymes : Their Importance And Classes
- Soil enzymes - South Dakota Soil Health Coalition
- Importance of soil enzymes
- History and Interpretation of Soil Enzyme Activity
Salinity
Salinity is a measure of the salt concentration in a soil. Saline soils are uncommon in Minnesota but may occur if a specific salt source exists (e.g. road salt, biosolid or manure application), in irrigated soils, and in arid soils.
Electrical conductivity (EC) is a good indicator of soil salinity. EC values greater than 16 dS/m indicate saline soils. If EC suggests saline soils, it may be useful to take laboratory samples to identify specific chemicals of concern (e.g. sodium, potassium).
Stormwater application: Salinity is not widely used in stormwater applications, but is a potentially useful indicator of soils impacted by road salts or other high salt materials.
Additional reading
- Salinization - USDA-NRCS
- Salty soils - FAO
- Measuring soil salinity
- Soil Salinity Testing, Data Interpretation and Recommendations
Sodium adsorption ratio

Sodium adsorption ratio (SAR) is a measure of the amount of sodium (Na+) relative to calcium (Ca2+) and magnesium (Mg2+) in water extracted from a saturated soil paste. Soils with SARs of 13 or more are considered sodic. Sodic soils may have increased dispersion of organic matter and clay particles, resulting in reduced saturated hydraulic conductivity and aeration and a general degradation of soil structure.
SAR is calculated knowing the concentrations of Na, Ca, and Mg in soil, which are measured in the laboratory. SAR is given by the formula
- SAR = (Na+) / ((Ca2+ + Mg2+)/2)1/2
where the chemicals represent concentrations in soil.
Stormwater application: SAR is not widely used in stormwater applications, but may be recommended in soils or engineered media impacted by road salt.
Additional reading
- Sodium Adsorption Ratio
- Sodium Adsorption Ratio and Sodicity - Soil Health Nexus
- Sodium Adsorption Ratio
pH
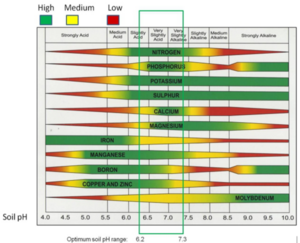
Soil pH is a measure of soil acidity or alkalinity. It is an important soil property that affects plant suitability, nutrient availability, soil microorganism activity, chemical cycling, and mobility of pollutants such as metals.
pH may be measured in the field or laboratory. Field test strips offer easy and quick methods for estimating soil pH, though they are not as accurate as other methods. Recommended lab methods include the following.
- Saturated paste - recommended
- 1:1 and 2:1 water ratios
Soil pH can be managed by measures such as applying the proper amount of nitrogen fertilizer, liming, and cropping practices that improve soil organic matter and overall soil health.
Stormwater application: pH is an important soil property affecting plant growth and fate of environmental pollutants. Most Minnesota soils have acceptable pH for most plant applications. In vegetated stormwater practices, soil pH should be determined prior to plant selection.
Recommended reading
- Soil pH - USDA-NRCS
- Soil pH - SUNY College of Environmental Science and Forestry
- Soil pH
- Changing the pH of your soil
- Soil Quality Indicators: pH - USDA-NRCS
- Understanding soil pH - Penn State University
Soil chemical processes
This section provides information on the more important soil chemical processes.
Precipitation, dissolution, chemical weathering and mineral formation
Chemical weathering is the transformation of minerals to solutes and solid residues. Weathering leads to formation of iron and aluminum oxides, clay minerals, and amorphous silicates. Weathering rates primarily vary with temperature, precipitation, and type of mineral, though other factors such as pH and oxidation-reduction can be important. Although weathering is a slow process, the secondary minerals formed from weathering have a significant effect on soil properties, including the fate of nutrients and pollutants.
Important precipitation and dissolution reactions in soil include but are not limited to the following.
- Dissolution of chloride salts (sodium, potassium, magnesium, and calcium chlorides)
- Dissolution of gypsum (calcium sulfate)
- Dissolution of aluminum hydroxides in acidic soils
- Precipitation of phosphorus in acidic and basic soils
- Precipitation of metals with organic compounds
Recommended reading
Redox (oxidation-reduction) processes

Oxidation-reduction reactions, also called redox, involves the transfer of one or more electrons between two chemicals. An example is the oxidation of carbon as organisms utilize the carbon as a food source. If oxygen is involved in this reaction, carbon is being oxidized (losing electrons) while oxygen is being reduced (accepting electrons).
Redox is typically expressed as Eh, which is the potential, in volts, of an inert (e.g. platinum) electrode measured relative to the standard hydrogen electrode. Higher Eh values represent more oxidized conditions. Electron acceptors in soil, in order from highest to lowest oxidation status, are oxygen, nitrate, manganese, iron, and sulfate. Thus, under moderately reducing conditions in the absence of oxygen, nitrate is the electron acceptor and is converted to nitrogen gas. Similarly, under more reducing conditions, iron is reduced. Redox status (Eh) in soil thus affects nutrient availability, adsorption of pollutants, metal mobility, and so on.
Redox conditions in soil can be manipulated. For example, nitrogen bioreactors are designed to promote reducing conditions where nitrate, a pollutant of concern for surface waters, is reduced. The primary management controls on redox are management of water and organic matter, both of which promote reducing conditions in soil. Stormwater systems may often be designed with oxic and anoxic zones to promote biological activity in one zone but retain pollutants of concern in the other layer.
For information on specific redox reactions of importance in soil, see the first reference below.
Recommended reading
- Redox Chemistry of Soils
- Redox Potential
- Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy
- Soil and Soil Solution Chemistry
- Identifying and characterizing redoximorphic features in soils and soil borings
Cation exchange
Cation exchange is the exchange of a cation in soil water solution of water with a cation sorbed to soil, typically clay or organic matter. Cations of most importance in soil are calcium, magnesium, potassium, sodium, and in acidic soils, aluminum. The sum of positive charges that the soil can absorb is called the cation exchange capacity (CEC).
CEC affects the nutrient status of soil. Soils with greater organic matter and/or clay content have higher CEC and typically have greater amounts of available nutrients than sandy soils. Soils with high sodium concentrations (sodic soils) will have a high percent of sodium as the exchangeable cation. Excess sodium is detrimental to soil structure. In acidic soils (pH < 5), aluminum becomes an exchangeable cation. Aluminum has no nutritional value for plants and can be toxic to plants.
Cation exchange can be manipulated by adding soil organic matter, which has a high CEC. In acidic soils, liming will raise pH and increase exchangeable calcium and magnesium.
Recommended reading
- Cation exchange capacity
- Cation exchange capacity - Cornell University
- Cation Exchange Capacity and Base Saturation - University of Georgia
- Calculating Cation Exchange Capacity, Base Saturation, and Calcium Saturation - The Ohio State University
Complexation
Complexation involves the combination of a metal ion with a compound containing chelate-forming, electron-donating functional groups to form metal chelates. Complexation reactions increase mobility of chemicals, particularly metals. Complexation reactions at the mineral–water interface therefore affect the transport and transformation of metals and organic contaminants, nutrient availability in soils, formation of ore deposits, acidification of watersheds and the global cycling of elements.
The primary chelating agents in soil are humic and fulvic acids, which form as organic matter breaks down. Thus, adding organic matter to soil, particularly aged (composted) organic matter, will increase complexation.
Recommended reading
- The significance of surface complexation reactions in hydrologic systems: a geochemist’s perspective
- Soil and Soil Solution Chemistry
- Organic Matter, Humus, Humate, Humic Acid, Fulvic Acid, and Humin: Their Importance in Soil fertility and Plant Health
Sorption and desorption, including precipitation
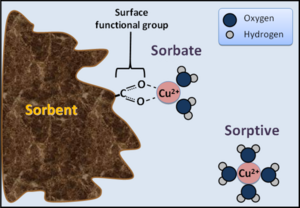
Sorption is removal of a compound from solution to a solid phase, while desorption is release of ions or molecules from soil solids into solution. Sorption includes the following.
- Adsorption: the accumulation of chemicals at the solid-liquid interface
- Absorption: the accumulation of molecules within existing solids as absorption
- Precipitation: the incorporation of substances within an expanding three-dimensional solid
Sorption can generally be divided into three categories.
- Anionic sorption involves positively charged surfaces attracting negatively charged chemicals (e.g. phosphate, nitrate, sulfate). At near neutral pH (6-8), anionic sorption is limited in most Minnesota soils. It becomes more important in acidic soils.
- Cationic sorption involves negatively charged surfaces attracting positively charged chemicals (e.g. calcium, magnesium, potassium, sodium). This is an important mechanism in most Minnesota soils, particularly in soils with high clay content.
- Uncharged surfaces may attract a range of chemicals depending on the nature of the surface. These primarily consist of organic materials. Polar chemicals, such as those having functional groups (e.g. phenols, amines, alcohols, carboxylic acids) attract chemicals with an opposite charge. At near neutral pH (6-8), most of these functional groups are negatively charged, thus leading to cation sorption. Non-polar chemicals, such as aromatic compounds, are effective at sorbing other non-polar compounds, such as petroleum products.
Sorption is affected by soil pH, with increased sorption of anionic compounds at low pH and cationic compounds at neutral and basic pH. Practices such as liming or adding acidifying agents therefore affects the mobility and availability of chemicals in soil, including nutrients and pollutants.
Sorption processes in soil are complex and beyond the scope of this discussion. The extent and strength of sorption is affected by the characteristics of the sorbing surface, abundance of specific ions in soil solution, and pH. See suggested reading below for more information.
Recommended reading
- Introduction to the Sorption of Chemical Constituents in Soils
- Chemical Properties of Soil
- Sorption-Desorption Processes of Metals and Metalloids in Soil Environments
- The Concept of Soil Adsorption and Desorption
Hydrolysis
Hydrolysis is the chemical breakdown of substances by water. Hydrolysis depends on the chemistry, solubility, pH, and the oxidation–reduction (Eh or redox) potential of a compound. A common type of hydrolysis occurs when a salt of a weak acid or weak base (or both) is dissolved in water.
Potentially important hydrolysis reactions in soil involve aluminum and phosphorus. Organic and condensed phosphate compounds in soil have to be hydrolysed to become available to plants and microorganisms. Aluminum reacts with water (hydrolysis) to form aluminum hydroxides. This process affects soil pH and concentrations of aluminum in soil water, which in turn can affect plant growth.
Recommended reading
This page was last edited on 1 December 2022, at 01:22.