Pollutant fate and transport in stormwater infiltration systems
This site is under construction. Anticipated completion date is May, 2015.
As stormwater travels across the land surface into infiltration BMPs, it can pick up various pollutants and deliver them to the subsurface. The fate and transport of these pollutants into soil, the vadose zone and ultimately groundwater depends on the type and amount of pollutant present, the volume of infiltration, the type of infiltration BMP, and subsurface conditions.
Contents
Typical stormwater pollutants
Common stormwater pollutants and their most important sources are described in the first table below. The second table provides typical pollutant concentrations in stormwater runoff. The concentrations are based on data from the International Stormwater Database.
Common pollutants of concern and sources in stormwater runoff. Adapted from USGS, 2014.
Link to this table.
| Contaminant | Contaminant source1 |
|---|---|
| Nitrogen | Naturally occurring from vegetation decomposition. Anthropogenic sources include fertilizers, farm-animal waste, faulty septic systems |
| Chloride | Salts applied to roads and parking lots during the winter. Natural sources include mineral dissolution |
| Copper | Industrial and domestic waste, mining, mineral leaching, automobile parts and fluids |
| Zinc | Industrial waste; automobile parts and fluids |
| Manganese | Found naturally in sediment and rocks. Anthropogenic sources include mining waste, industrial waste, automobile parts and fluids |
| Nickel | Naturally occurring. Anthropogenic sources include stainless steel and alloy products, mining, refining, automobile parts and fluids |
| Cadmium | Small amounts are naturally occurring. Anthropogenic sources include industrial discharge, mining waste, automobile parts and fluids |
| Chromium | Old mining operations; fossil-fuel combustion; mineral leaching; automobile parts and fluids |
| Pesticides | Residential use of lawn care products; commercial landscaping; animal wastes; municipal right-of-ways; agriculture; feedlots |
| Cyanide | Road salt; fertilizer production |
| PAHs2 | Auto emissions; elicit discharges; asphalt pavement (driveways, roadways and parking lots) with coal tar sealants3 |
| VOCs2 | Crude oil; insecticides; varnishes; paints; gasoline products; degreasers; municipal maintenance activities |
| Oil and grease | Gasoline products; plastics; dyes; rubbers; polishes; solvents; crude oil; insecticides; inks; varnishes; paints; disinfectants; paint removers; degreasers; automobile fluids |
| Microbes (including fecal coliform, E. coli, and pathogens) | Domestic sewage; animal waste; plant or soil material |
1The list of sources is for stormwater runoff only
2PAHs=polyaromatic hydrocarbons; VOCs=volatile organic compounds
3MPCA, 2014
Source: USGS, 2014, with permission
Concentrations of contaminants found in stormwater. Source: International Stormwater Database7. Because the data below are from a single source, values may differ from those contained on this page. We recommend if you are using emcs to quantify pollutant loading, you use this data instead of data from this table. Note that the table does not include information for chloride, a common pollutant in stormwater. Chloride concentrations vary seasonally and would be misrepresented in a single table. For more information on chloride concentrations in stormwater, see here.
Link to this table.
| Land use | TSS 1 | NO2 + NO3 1 | TN 1 | TP 1 | Cu 2 | Zn 2 | Ni 2 | Cd 2 | Cr 2 | CN 2,5 | Oil and grease 2 | VOCs 2,5 | Pesticides 2,4,5 | FC 3,5 | EC 3,5 | FS 3,5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Commercial | ||||||||||||||||
| Number of sites | 56 | 50 | 13 | 56 | 60 | 62 | 40 | 51 | 38 | 2 | 44 | 4 | 1 | 4 | -- | 3 |
| Number of observations | 857 | 786 | 77 | 948 | 785 | 867 | 291 | 543 | 294 | 6 | 394 | 160 | 6 | 19 | -- | 7 |
| % of samples above detection | 98.7 | 98.9 | 97.4 | 94.5 | 85 | 99.2 | 51.5 | 38.1 | 52.0 | 0 | 65.5 | 65.5 | 0 | 73.7 | -- | 100 |
| Minimum | <0.5 | <0.1 | <1.5 | <0.01 | <0.2 | <0.3 | <1 | <0.03 | <0.7 | n/a | <0.5 | <0.05 | n/a | <200 | -- | 310 |
| Maximum | 2385 | 8.2 | 18.1 | 4.27 | 569.1 | 3050.5 | 110 | 80 | 100 | n/a | 359 | n/a | 28000 | -- | 24000 | |
| Median | 52 | 0.6 | 1.75 | 0.2 | 17 | 110 | 8 | BDL6 | 4 | BDL | 5 | 0.7 | n/a | 450 | -- | 3100 |
| Industrial | ||||||||||||||||
| Number of sites | 58 | 51 | 13 | 57 | 65 | 67 | 43 | 60 | 42 | 2 | 48 | 3 | -- | 6 | -- | 4 |
| Number of observations | 619 | 536 | 85 | 638 | 569 | 627 | 300 | 525 | 312 | 9 | 370 | 144 | -- | 32 | -- | 12 |
| % samples above detection | 99.5 | 97.0 | 95.3 | 95.1 | 85.1 | 98.9 | 58.0 | 48.6 | 72.4 | 0 | 59.7 | 10.4 | -- | 90.6 | -- | 91.7 |
| Minimum | <1 | <0.02 | <1.5 | <0.02 | <0.2 | <0.5 | <2 | <0.03 | <0.7 | n/a | <0.5 | <0.05 | -- | <1 | -- | <1 |
| Maximum | 2490 | 8.4 | 15.2 | 7.9 | 1360 | 8100 | 120 | 334 | 150 | n/a | 408 | -- | 3600000 | -- | 48000 | |
| Median | 75 | 0.68 | 1.7 | 0.23 | 19 | 155 | 10 | BDL | 10 | BDL | 5 | BDL | -- | 3950 | -- | 24000 |
| Residential | ||||||||||||||||
| Number of sites | 146 | 127 | 20 | 148 | 147 | 151 | 77 | 114 | 72 | -- | 95 | 7 | 1 | 10 | 3 | 4 |
| Number of observations | 2257 | 1772 | 131 | 2380 | 1743 | 2013 | 418 | 1123 | 408 | -- | 694 | 210 | 6 | 94 | 19 | 23 |
| % of sample above detection | 99.9 | 99.0 | 98.5 | 98.2 | 86.5 | 97.0 | 42.2 | 40.4 | 48.8 | -- | 56.8 | 20.1 | 0 | 85.9 | 100 | 95.7 |
| Minimum | <0.5 | <0.03 | <1.5 | <0.01 | <0.2 | <0.5 | <0.5 | <0.03 | <0.7 | -- | <0.5 | <0.05 | n/a | <1 | 10 | <1 |
| Maximum | 4168 | 66.4 | 18.3 | 19.90 | 590 | 14700 | 100 | 70 | 70 | -- | 419 | 3.42 | n/a | 5230000 | 35000 | 200000 |
| Median | 58 | 0.60 | 2.24 | 0.26 | 11 | 69.9 | 5 | BDL | 3 | -- | 4 | BDL | BDL | 9400 | 1000 | 23500 |
| Open space | ||||||||||||||||
| Number of sites | 15 | 13 | 4 | 15 | 12 | 12 | 9 | 8 | 7 | 3 | 9 | 1 | -- | 2 | 1 | -- |
| Number of observations | 105 | 109 | 13 | 111 | 44 | 49 | 38 | 41 | 36 | 13 | 26 | 5 | -- | 6 | 5 | -- |
| % of samples above detection | 97.1 | 92.7 | 92.3 | 93.7 | 64.4 | 65.3 | 23.1 | 39.0 | 36.1 | 15.4 | 34.6 | 60.0 | -- | 100 | 100 | -- |
| Minimum | <1 | <0.1 | <0.5 | <0.01 | <0.8 | <5 | <2 | <0.04 | <0.7 | <0.01 | <1 | <0.2 | -- | 1900 | 100 | -- |
| Maximum | 4168 | 3.4 | 3.3 | 0.76 | 210 | 390 | 100 | 8 | 120 | 0.08 | 11 | 0.84 | -- | 63000 | 4700 | -- |
| Median | 58 | 0.5 | 1.1 | 0.129 | 6 | 25 | BDL | BDL | BDL | BDL | BDL | 0.77 | -- | 2150 | 1100 | -- |
| Rooftop | Water quality from rooftops varies with the type of roof. For more information see the section on Water quality considerations for stormwater and rainwater harvest and use/reuse | |||||||||||||||
TSS=total suspended solids, NO2=nitrite, NO3=nitrate, TN=total nitrogen, Cl=chloride, Cu=copper, Zn=zinc, Ni=nickel, Cd=cadmium, Cr=chromium, CN=cyanide, VOC=volatile organic compound, FC=fecal coliform, EC=E. coli, FS=fecal streptococci
1 Concentrations are in milligrams per liter
2 Concentrations are in micrograms per liter
3 Concentrations are in Number per 100 milliliters
4Data is for trans-1,3-Dichloropropene and bromomethane
5 Data was selected from states with a similar climate to MN. The appropriate states were determined using Figure 1.3 from the Stormwater BMP Design Supplement for Cold Climates document.
6BDL = below detection level
7The following censoring techniques were used for this data:
- If detection rates were 90% or greater, a value of "0" was substituted for non-detects.
- If detection rates were greater than 50% but 90% or less, a value of 1/2 the detection limit was substituted for non-detects.
- If detection rates were 50% or less, the median was assumed to be below the method detection level.
Nitrogen in stormwater
| Summary of characteristics of nitrate-nitrogen. Sources:Pitt et al., 1994, 1999; Weiss et al., 2008; ATSDR, 2011. | |
| Mobility | Mobile |
| Solubility | High |
| Abundance in stormwater | Low/moderate |
| Toxicity | Low. Primary concern is for infants less than 6 months in age. |
| Degradation potential | High in anaerobic environments; low in aerobic environments |
| Adsorption/absorption | Low |
| Plant uptake | High |
| Potential risk to groundwater | Low/moderate based on high mobility but relatively low concentrations in urban stormwater. |
For an excellent review of nitrogen, link here. The following discussion provides a general overview of nitrogen in stormwater and fate and transport in soil and the vadose zone.
Nitrogen is found in many forms in stormwater runoff, with the most common forms being ammonium+ammonia, organic nitrogen, nitrate, and nitrite. Detectable concentrations of nitrogen occur in more than 95 percent of samples collected from urban runoff (International Stormwater Database). Total nitrogen concentrations in urban stormwater are typically in the 1 to 2 milligram per liter range. Concentrations tend to be somewhat higher in residential areas compared to other land uses (International Stormwater Database).
Ammonium, ammonia, and organic nitrogen comprise the reduced forms of nitrogen and typically account for about two-thirds of total nitrogen in stormwater runoff, although this varies widely with source area (see EPA). Together, these forms are expressed as Total Kjeldahl nitrogen. These forms of nitrogen have low mobility and are attenuated in most stormwater management BMPs through adsorption or oxidation to nitrate.
Nitrate is highly mobile in aerobic environments. It is estimated to be the most common nonpoint-source groundwater contaminant in the world (Gurdak & Qi, 2012). Despite its high solubility, nitrite is detected with much less frequency than nitrate because nitrite oxidizes rapidly to form nitrate. Nitrate concentrations in stormwater are typically 1 milligram per liter or less, well below the drinking water standard of 10 milligrams per liter.
Ammonia is highly toxic to humans and aquatic organisms, but concentrations in stormwater are typically well below levels of concern. Nitrate has relatively low toxicity, although concentrations exceeding 10 milligrams per liter in drinking water can lead to the phenomenon known as “blue baby syndrome” which affects babies less than 6 months old (Prey et al., 2000). Nitrates and nitrites have not been classified as carcinogenic, however a metabolic pathway exists that lead to formation of N-nitroso compounds, some of which are carcinogenic (ATSDR, 2011).
Chloride in stormwater
| Summary of characteristics of chloride. Sources:Pitt et al.; Neiber et al., 2014. | |
| Mobility | Mobile |
| Solubility | High |
| Abundance in stormwater | Seasonal high (winter, early spring) |
| Toxicity | Low |
| Degradation potential | Low |
| Adsorption/absorption | Low |
| Plant uptake | Low |
| Potential risk to groundwater | High |
Chloride in stormwater often comes from the salts used in road surface deicing agents. Only limited data was found in the International Stormwater Database for chloride concentrations in stormwater runoff. Chloride was detected in approximately 38 percent of the 29 samples that were submitted to the database. Only sites in northern climates were included in this analysis.
Chloride is highly mobile in soil and will readily leach through the vadose zone and into groundwater.
At elevated concentrations, chloride can become toxic to aquatic life. Elevated levels of chloride can also result in low oxygen conditions, leading to the release of phosphorous and metals sorbed to the solids (Novotny et al., 2008). In addition, high levels of chloride will increase the density of the water, causing the salt containing water to settle to the bottom of the water body. This results in stratification and disrupts lake mixing patterns (New Hampshire Department of Environmental Services).
Cyanide in stormwater
| Summary of characteristics of cyanide. Sources:ATSDR, 2006; EPA Technical Factsheet: Cyanide, N.D. | |
| Mobility | Mobile |
| Solubility | Depends on form of cyanide. |
| Abundance in stormwater | Seasonal (highest in winter, early spring) |
| Toxicity | High |
| Degradation potential | Moderate if not at toxic concentrations |
| Adsorption/absorption | Nitriles and soluble cyanides (e.g. H- and K-cyanide) have low absorption potential. Insoluble forms may sorb to soil particles. |
| Plant uptake | Low |
| Potential risk to groundwater | Low based on low concentrations in stormwater runoff |
Cyanide is often found in road salt, where it is used as an anti-caking agent. Another source of cyanide is discharge from industrial facilities. Only limited data was found in the International Stormwater Database for cyanide concentrations in stormwater runoff. Cyanide was detected in approximately 10 percent of the 23 samples submitted to the Database. As with chloride, only sites in northern climates were included.
Mobility in soil depends on the form of cyanide. Nitriles have the potential to leach to ground water as they do not adsorb to soil. They tend to be resistant to hydrolysis in soil or water. Cyanide-containing herbicides have more moderate potential for leaching. Soluble cyanide compounds such as hydrogen and potassium cyanide have low adsorption to soils with high pH, high carbonate and low clay content. At pH less than 9.2, most free cyanide is expected to convert to hydrogen cyanide, which is highly volatile. Soluble cyanides are not expected to bioconcentrate. Insoluble cyanide compounds such as the copper and silver salts may adsorb to soils and sediments EPA.
Cyanide is an extremely toxic pollutant. It prevents the body from using oxygen and at a sufficient concentration it can lead to death. Low exposure can cause headache or dizziness (ATSDR, 2006). Chronic exposure can lead to nerve damage or thyroid problems.
Metals in stormwater
| Summary of characteristics of metals. Sources:Pitt et al., 1994; Weiss et al., 2008; ATSDR, 2004, 2005, 2007, 2012 | |
| Mobility | Very low/intermediate |
| Solubility | Low |
| Abundance in stormwater | Low/high |
| Toxicity | Variable (see discussion in text) |
| Degradation potential | Low |
| Adsorption/absorption | High |
| Plant uptake | Low |
| Potential risk to groundwater | Low, except possibly for zinc |
A nationwide analysis of stormwater runoff by Pitt et al. (2004) found one or more metal in almost all samples tested. Of primary concern are cadmium, copper, lead, and zinc (Nieber et al., 2014). The aforementioned metals were detected at varying frequencies in the sites that were reported in the International Stormwater Database. Cadmium was detected in 42 percent of 2,234 samples, copper in 86 percent of 3,125 samples, lead in 75 percent of 2,667 samples, and zinc in 98 percent of 3,552 samples.
Metals typically have low solubility and are not mobile in soil. Most are readily adsorbed within typical pH ranges found in soil. Mobility typically increases for most metals as pH decreases. metals may also form complexes with organic matter and these may increase their mobility.
At trace concentrations, several of these metals are essential to human life. At higher concentrations they can be toxic. Cadmium has the potential to bioaccumulate in the ecosystem, and at high enough concentrations, it can kill aquatic life. In humans, cadmium has been found to lead to kidney damage (ATSDR, 2012). Copper is toxic to both human and animal life. Short term exposure often results in gastrointestinal distress while long term exposure can lead to kidney damage. People with Wilson’s Disease are especially vulnerable to the effects of copper (ATSDR, 2004). Lead toxicity targets the nervous system and long term exposure may result in a decreased performance in tests that measure the function of the nervous system. Lead can also result in anemia or a small increase in blood pressure. At high concentrations, lead can damage the brain and kidneys (ATSDR, 2007). Zinc is toxic to aquatic life and can effect human health if it is ingested at levels 10 to 15 times higher than the amount needed for general health. Zinc can cause stomach cramps, nausea, and vomiting. Long term exposure can lead to anemia and a decrease in good cholesterol. Zinc may also have an effect on reproduction, though this has not been confirmed (ATSDR, 2005).
Pesticides in stormwater
| Summary of characteristics of pesticides. Sources: Pitt et al., 1994 | |
| Mobility | Intermediate/mobile |
| Solubility | Variable |
| Abundance in stormwater | Low/moderate |
| Toxicity | Generally high |
| Degradation potential | Variable. Ranges from days to years. |
| Adsorption/absorption | Variable; generally moderate/high |
| Plant uptake | Herbicides are more likely to be taken up by plants than insecticides |
| Potential risk to groundwater | Variable, mainly low to moderate. Fungicides and nematocides are the most mobile (Pitt et al., 1999). See Tables 2 and 3 in Pitt et al. (1999) for the mobility potential of several common pesticides (type in full address to access pdf document: http://www.researchgate.net/profile/Shirley_Clark2/publication/222468641_Groundwater_contamination_potential_from_stormwater_infiltration_practices/links/00b7d523c9e3b35d0a000000.pdf). |
Pesticides in stormwater runoff come from land application of insecticides, herbicides, fungicides, rodenticides, and algaecides. Only limited data was found for pesticides in the International Stormwater Database. Pesticides were not detected in any of the 12 samples submitted to the Database. In a summary of studies and sampling events presented in Pitt et al., (1994), pesticides such as diazionon, Malathion, 2,4-D, fungicides, dacthal, as well as many others were detected in stormwater runoff. A 1990 study by the EPA found 46 pesticides in the groundwater of 35 states. In Minnesota specifically, 14 types of pesticides were detected (Pitt et al., 1994).
Pesticides have been linked to cancer, birth defects, nerve damage, and many other disorders. Toxicity varies widely among pesticides, with insecticides typically being more toxic than herbicides. Oklahoma State University produced a report summarizing toxicity for major pesticides.
Polycyclic Aromatic Hydrocarbons (PAHs) in stormwater
| Summary of characteristics of pesticides. Sources: Pitt et al., 1994 | |
| Mobility | Low/intermediate; decreases with increased molecular weight |
| Solubility | Low/intermediate; decreases with increased molecular weight |
| Abundance in stormwater | Varies |
| Toxicity | Generally high |
| Degradation potential | Variable. The potential for degradation depends on the types of microorganisms present in the soil as well as they type of contaminant. See Haritash and Kaushile (2009) for a review of the biodegradation process. |
| Adsorption/absorption | High |
| Plant uptake | Low |
| Potential risk to groundwater | PAHs with the lower molecular weight have a moderate to high transportation potential while the heavier PAH have a low potential. See Tables 2 and 3 in Pitt et al. (1999) for the mobility potential of several common PAHs (type in full address to access pdf document: http://www.researchgate.net/profile/Shirley_Clark2/publication/222468641_Groundwater_contamination_potential_from_stormwater_infiltration_practices/links/00b7d523c9e3b35d0a000000.pdf). |
Polycyclic Aromatic Hydrocarbons (PAHs) are compounds consisting of two or more aromatic rings. They are found naturally in soils but are also created by the incomplete combustion of organic chemicals. Common examples of PAHs include napthlene, benzo(a)pyrene, chrysene, and pyrene. While the health risks vary with the different types of PAHs, they are generally toxic (ASTDR, 1995). Toxicity typically increases with increasing molecular weight and even small concentrations of the most toxic PAHs are a concern. No information was given in the International Stormwater Database on their occurrence in stormwater. Hwang and Foster (2006) found stormwater runoff concentrations in the part per billion (microgram per liter) range, with automobiles being the primary source. Coal tar sealants have also been shown to be an important contributor to PAHs in stormwater runoff (Watts et al., 2010; Mahler et al., 2012.)
The health effects of PAHs will depend on the type of PAH present. Benzo(a)pyrene for example causes reproductive difficulties and leads to an increased risk of cancer. In general, PAHs with a higher molecular weight are more toxic, less soluble, and more persistent in the environment.
Volatile Organic Compounds (VOCs) in stormwater
| Summary of characteristics of pesticides. Sources: Pitt et al., 1994 | |
| Mobility | Mobile |
| Solubility | Variable |
| Abundance in stormwater | Low |
| Toxicity | Generally high |
| Degradation potential | Variable. It is dependent on the type of VOC, presence of certain microbial species, availability of carbon sources, and environmental conditions. |
| Adsorption/absorption | Low |
| Plant uptake | Low |
| Potential risk to groundwater | Moderate |
Volatile Organic Compounds (VOCs) are a large group of carbon-based chemicals that easily evaporate at room temperature Minnesota Department of Health. Some common groups of VOCs include the following.
- BTEX (benzene, toluene. ethylbenzene, xylene) compounds, commonly found in gasoline, paints, and solvents.
- Halogenated compounds, which are organic compunds containing fluorine, chlorine, bromine, or iodine. Chlorinated compounds are the most common of these and they are widely used as solvents and degreasers.
- non-halogenated compounds, which includes a large number of compounds with a wide variety of uses. Examples include ketones (e.g. acetone), alcohols (ethanol), and ethers (methyl tert-butyl ether). Fairly limited data on VOC were found in the International Stormwater Database. VOC were detected in 31 percent of the 519 samples submitted to the Database
The health effects of VOCs vary depending on the type present and exposure pathway. Exposure can occur via inhalation or ingestion. Often times VOCs effect the nervous system, kidneys and/or liver. Some are carcinogens, while other may cause irritation when in contact with the skin (MN Department of Health, N.D.). The EPA provides a summary of health effects from ingestion for many of the more common VOCs.
Pathogens in stormwater
Pathogens, or disease causing organisms, can be broken into three categories: bacteria, protozoa, and viruses. Examples of the different types of pathogens are shown below.
Pathogen types and examples (adapted from Urban Waterways: Removal of Pathogens in Stormwater, North Carolina, N.D.)
Link to this table.
| Type | Example pathogens (disease) |
|---|---|
| Bacteria | Salmonella (Salmonellosis), Escherichia coli (E. coli) O125:H7 (Gastroenteritis), Vibrio cholera (Cholera), and Salmonella typhi (Typhoid fever) |
| Protozoa | Giardia lamblia (Giardiasis), Cryptosporidium (Cryptosporidiosis), Entamoeba histolytica (amoebic dysentery) |
| Virus | Hepatitis A (infectious hepatitis), Rotavirus (Gastroenteritis, Adenovirus (respiratory disease, gastroenteritis) |
Many of these pathogens are commonly found in runoff and may pose a threat to human health. Fecal coliform and E.coli bacteria are the most commonly used indicators of pathogen presence. E. coli was found in 100 percent of the 24 samples submitted to the International Stormwater Database. Fecal coliforms were detected in 86 percent of the 151 samples submitted to the Database. Clark and Pitt (2007) found fecal streptococci and E. coli in 94 and 95.5 percent, respectively, of the municipal separate storm sewer outfalls they tested (presented in Nieber et al., 2014). Select pathogens are discussed in further detail below. Please note that the mobility of these pathogens is based on the worst case scenario (i.e. sand/low organic soils).
- Coliforms (including fecal coliforms and E. coli)
Coliforms include fecal coliforms and E. coli. Fecal coliform is a bacteria associated with both human and animal waste. E. coli is a subset of the fecal coliforms. Overall, most strains of E. coli are harmless, although some are in fact pathogenic. Those that are pathogenic may cause, among other things, diarrhea, urinary tract infections, respiratory illness, and pneumonia. Fecal coliforms and E. coli are often used as indicator organisms because their presence often indicates that other, more pathogenic organisms are present. There is growing evidence, however, that these organisms might not correlate well with the presence or absence of viruses and other pathogens. Some people are moving towards using total coliforms, though E. coli and fecal coliforms are still common. Below is a list of some of the characteristics of coliforms (Clark and Pitt, 2007, as presented in Nieber et al., 2014; some information is based on characteristics of other bacteria in Pitt et al., 1994):
- Abundance in stormwater: Likely present
- Mobility: Low/intermediate
- Potential transport to groundwater: Low/moderate
- Giardia lamblia (Giardia)
Giardia is a protozoan pathogen that causes gastrointestinal illness if ingested. Specifically, it can cause diarrhea, stomach or abdominal cramps, upset stomach or nausea, and dehydration (CDC, 2012). Below is a list of some of the characteristics of Giardia (Based on protozoa characteristics provided in Pitt et al., 1994):
- Abundance in stormwater: Likely present
- Mobility: Low/intermediate
- Potential transport to groundwater: Low/moderate
- Cryptosporidium
Like Giardia, Cryptosporidium is a protozoan pathogen that causes gastrointestinal illnesses if ingested. Common symptoms are stomach or abdominal cramps, nausea, severe diarrhea, and dehydration. If a person is immuno-compromised, this pathogen can be fatal. Below is a list of some of the characteristics of Cryptosporidium (Based on protozoa characteristics provided in Pitt et al., 1994):
- Abundance in stormwater: Likely present
- Mobility: Low/intermediate
- Potential transport to groundwater: Low/moderate
- Enterovirus
The group Enterovirus is made up of many different viruses, including polio. Often times a person who becomes infected with a non-polio Enterovirus does not become sick. Of those that do, common symptoms range from symptoms similar to the common cold up to an infection of the heart or brain which could lead to paralysis. Below is a list of some of the characteristics of Enterovirus (Pitt et al., 1994):
- Abundance in stormwater: Likely present
- Mobility: High
- Potential transport to groundwater: High
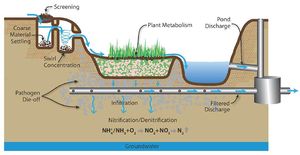
Pollutant fate and transport
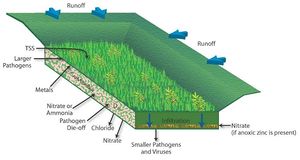
A concern with infiltration of stormwater runoff is the potential transport of pollutants in stormwater through soil and into groundwater, where they may impact drinking water supplies or surface water when discharged to lakes, rivers or wetlands. Many pollutants are attenuated in stormwater control practices (BMPs), in soil or the vadose zone, or in groundwater. Some pollutants are poorly attenuated, however, and these represent a potential risk.
In general, particulate pollutants (such as total suspended solids [TSS]) and those pollutants that primarily bind to particulates (such as metals) are easily removed by the filtration process within the infiltration BMPs. Soluble contaminants on the other hand, such as chloride, have the potential to be carried for some distance and may eventually reach the groundwater table. Protozoa and larger bacteria are more easily removed from the system than smaller bacteria and viruses. Of greatest concern are mobile toxic organics (gasoline, solvents), nitrates, viruses and chloride. If it is possible to do so, these contaminants should be removed from the stormwater prior to infiltration. To accomplish this, an appropriate pretreatment technique is needed. Any runoff containing toxic material that will not bind to soils, be easily removed, or excess volume that cannot infiltrate, should be diverted away from the infiltration BMP to another treatment device.
This section discusses the process of attenuation and includes an overview of the fate and transport of the most common pollutants in stormwater runoff.
Summary of attenuation-removal mechanisms for primary pollutants found in stormwater runoff. Adapted from Fundamentals of Urban Runoff Management: Technical and Institutional Issues, 2nd Ed.
Link to this table
| Mechanism | Pollutants affected | Promoted by |
|---|---|---|
| Sedimentation | Solids, BOD, pathogens, particulate COD, P, N, metals, synthetic organics | Low turbulence |
| Filtration | Solids, BOD, pathogens, particulate COD, P, N, metals, synthetic organics | Fine, dense herbaceous plants, constructed filters |
| Soil incorporation | All | Medium-fine texture |
| Precipitation | Dissolved P, metals | High alkalinity |
| Sorption - adsorption | Dissolved P, metals, synthetic organics | High soil Al, Fe, high soil organics (Met), circumneutral pH |
| Sorption - ion exchange | Dissolved metals | High soil cation exchange capacity |
| Oxidation | COD, petroleum hydrocarbons, synthetic organics | Aerobic conditions |
| Degradation - photolysis | COD, petroleum hydrocarbons, synthetic organics | Direct sunlight |
| Degradation - volatilization | Volatile petroleum hydrocarbons, synthetic organics | High temperature and air movement |
| Degradation - biological | BOD, COD, petroleum hydrocarbons, synthetic organics | High temperature and air movement |
| Plant metabolism | P, N, metals | High plant surface area |
| Pathogen die off | Pathogens | Plant excretions |
| Nitrification | Ammonia | Dissolved oxygen >2 mg/L, low toxicants, temperature >5-7°C, circumneutral pH |
| Denitrification | Nitrate, nitrite | Anaerobic, low toxicants, temperature >15°C |
Process of natural attenuation
Subsurface soil has the ability to remove some types of pollutants through a process called natural attenuation. Natural attenuation refers to the “reduction in mass or concentration of a compound in groundwater over time or distance from the source of constituents of concern due to naturally occurring physical, chemical, and biological processes, such as; biodegradation, dispersion, dilution, adsorption, and volatilization” (ASTM, 2003). It is emerging as a viable, and in some cases the preferred remedy for dealing with contaminated groundwater. It is often the preferred remedy because natural attenuation does not transfer the pollutants from one location to another but rather breaks them down in place, often into non-toxic end products.
The breakdown is often times performed by bacteria that naturally inhabit many of the ground water environments, or by die-off, such as with pathogens. More and more it is being discovered that bacteria are able to break down chemicals once thought to be virtually non-biodegradable. For example, components of gasoline such as benzene, toluene, ethylbenzene, and xylene (BTEX) are now known to biodegrade in ground water to carbon dioxide and water. Other contaminants, including chlorinated solvents (e.g., dry-cleaning solvents), can also biodegrade under particular conditions. In some cases, natural biodegradation may break down contaminants in ground water faster than they can be removed by engineered systems. Other types of removal that can occur include filtration, adsorption, and sedimentation.
Natural attenuation is not always a completely effective remedy by itself. In cases where the contamination is spreading more quickly than it can naturally break down, where drinking-water wells are in close proximity, where the characteristics of the pollutant does not lend itself to natural attenuation, or when toxic breakdown products occur, engineered systems are needed. Whether or not a pollutant will be removed from the stormwater runoff is determined by the characteristics of the chemical and subsurface conditions.
Nitrogen
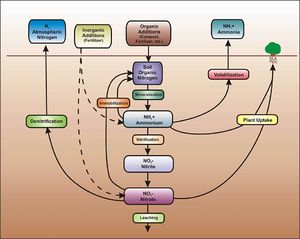
The primary forms of nitrogen in soil are organic nitrogen, ammonium, and nitrate. Nitrate is typically mobile in soil and is readily leached through soil and into the vadose zone. Organic nitrogen and ammonium are relatively immobile in soil but can be transformed to nitrate. Thus, when nitrogen containing compounds are present in the subsurface, there is a potential for nitrate to contaminate groundwater.
The fate of nitrogen in soil is affected by oxidation-reduction reactions. In addition, nitrogen in the root zone can be taken up by plants. In the vadose (unsaturated) zone, ammonium and ammonia undergo nitrification to become nitrite (NO2-) and then nitrate (NO3-). This process requires aerobic bacteria. If an anoxic zone is present, denitrification may occur within the infiltration BMP. When undergoing denitrification, the nitrate is reduced to molecular nitrogen (N2) and is lost to the atmosphere (WEF, 2012). If no anoxic zone is present, or the right bacteria are not present, nitrate will leach. Other removal mechanisms for nitrogen besides nitrification and denitrification are sedimentation and plant metabolism.
Due to relatively low concentrations in stormwater runoff, nitrate has only a low to moderate potential to contaminate the groundwater. BMPs that lack an engineered media with organic material and that allow stormwater runoff to infiltrate quickly will be most susceptible to nitrae leaching. These include infiltration basins and trenches, underground infiltration systems, and permeable pavement.
Chloride
Chloride has a high potential to contaminate groundwater. It is soluble, non-filterable, and does not sorb to solids (Levelton Consultants Ltd., 2008). There is no effective removal mechanism for chlorides. In addition, concentrations in stormwater runoff can be very high during winter and early spring, particularly in land use settings where road salt is used extensively. Rather than being removed, chloride concentrations are usually found to increase as infiltrated stormwater passes through the soil as naturally-occurring chloride is also removed from soil (Pitt et al., 1999).
Cyanide
At the soil surface, cyanide compounds will form hydrogen sulfide and evaporate into the atmosphere. In the soil, cyanide is fairly mobile but at low concentrations will most likely biodegrade under aerobic and anaerobic conditions (ATSDR, 2006).
Metals
Metals can be found in a dissolved state or bound to suspended solids in stormwater, with most of the metal mass bound to suspended solids. As such, metals are easily filtered out in soil and engineered media. Metals are not biodegraded, so they can accumulate and persist for long periods of time (Weiss et al., 2008). Unless the soils are disposed of, they will continue to accumulate.
Shanstrom (2014) provided an overview of metal fate and transport in bioretention systems. This overview was a review of peer-reviewed research. Breakthrough times, the time when metal concentrations in leachate from bioretention systems would reach levels of concern, was on the order of decades. this exceeds the typical life expectancy of the stormwater practice. Similarly, buildup of metals in the bioretention media to levels of concern ranged from about 25 years to several decades, depending on the metal. Again, this will likely exceed the life expectancy of the BMP. infiltration systems lacking engineered media with organic material may experience shorter time for breakthrough.
Leaching of metals may increase under certain environmental conditions. For example, mobility of most metals increases as soil pH decreases. If metals remobilize, or they are not filtered out near the surface to begin with, they will move further into the subsurface where a decrease in potential bonding sites has been observed (Hossan et al., 2007). This decrease in bonding sites makes it more likely that the metals will reach the groundwater.
Pitt et al. (1994) states that nickel and zinc have the highest groundwater contamination potential, while chromium and lead have only a moderate potential. This is based on their adsorption potential, which is provided below (Nieber et al., 2014).
- lead > copper > nickel > cobalt > zinc > cadmium
Other common removal mechanisms are precipitation, ion exchange, and plant metabolism. For plant accumulation, the tendency of the metals to be taken in by the plant are provided below, with zinc having the highest tendency (Sun and David, 2007, as reported in Nieber et al., 2014).
- zinc > copper > lead > cadmium
Pesticides
The majority of pesticides appear to be broken down via microbial degradation, however, some such as DDT, can persists for years (Balovsek, n.d.). Groundwater contamination will occur when the residence time of the pesticide in the vadose zone is less than that of the time it takes the pesticide to be broken down or transformed (Pitt et al., 1994). Pitt et al. (1994) concluded that those pesticides with half-lives greater than 30 days will pose the greatest risk to the groundwater. See Armstrong and Llena (1992) (as presented in Pitt et al., 1994) for more information on pesticide fate and transport.
PAHs
In general, PAHs with a higher molecular weight are more toxic, less soluble, and more persistent in the environment. PAH removal is not fully understood or as well studied as other pollutants (Weiss et al., 2008). Common removal mechanisms are oxidation, photolysis, volatilization, and biological degradation.
VOCs
VOCs have a high vapor pressure meaning they volatilize quickly. If the VOCs reach the subsurface and groundwater, much of the removal will come from degradation. A detailed description of the properties of the various VOCs, and well as their degradation potentials, can be found in the 2006 USGS report (Lawerence, 2006).
Pathogens
In general, protozoa and larger bacteria pose only a low to intermediate risk to groundwater contamination. This is due to their larger size, which allows them to be filtered out near the surface. Viruses on the other hand are much smaller and have a greater ability to survive in the subsurface, and therefore pose more of a threat to groundwater. Table 1.4 provides more information on pathogen removal in the subsurface.
Factors affecting the fate and transport of pathogens within the subsurface
Link to this table.
| Factor | Description | Source |
|---|---|---|
| Soil texture | Filtration is generally more effective in fine grained soils (i.e. silts and clays). This process is generally only significant when the average size of the pathogen is greater than 5% of the average pore space meaning protozoa and some bacteria may be removed this way, but not viruses. | Karathanasis et al., 2007; Ginn et al., 2002 |
| Soil cation exchange capacity | The process by which the pathogen becomes attached to the soil particles. It is most effective with viruses and smaller bacteria. Adsorption generally increases as the clay content in the soil increases and increases as soil pH decreases. | Bitton and Gerba, 1994; Lewis et al., 1980 |
| Soil moisture content | Moisture content appears to be one of the most influential factors in determining the survival time of pathogens. In general the survival time will increase as the moisture content increases. | Beard, 1940; Kibby et al., 1987 |
| Temperature | In general, survival time increases as temperature decreases. | McFeters and Stuart, 1972; Kibby et al., 1978 |
| pH | Survival time of pathogens appears greatest when soil is near neutral pH and decreases as the pH moves away from that range. | McFeters and Stuart, 1972 |
| Organic content | The increased presence of organic content increases the survival time of pathogens, as well as allows for the potential for some re-growth. This may be due not only to the presence of available nutrients but also to the fact that organic matter has the ability to retain moisture. | Lewis et al., 1980; Tate, 1978 |
| Predation | Microbial predators in the sub-surface reduce the concentration of pathogens in the soil and water (Tate, 1978). | Tate, 1978 |
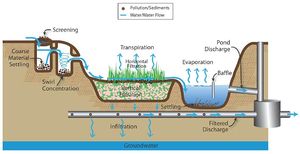
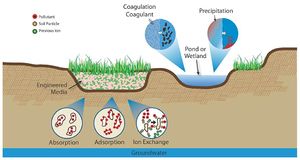
Mechanisms of pollutant removal in BMPs
The following table provides a summary of unit processes for BMPs designed for volume control, treatment through physical processes, treatment through biological processes, and treatment through chemical processes. These unit processes are described below in greater detail.
Unit processes of stormwater pretreatment techniques (Adapted from WEF, 2008)
Link to this table
| Control | Vegetated filter strips | Vegetated swale | Forebays | Street/parking lot sweeping | Proprietary settling/swirl chambers | Oil/water separators | Green roofs | Cisterns | Drain inlet inserts |
|---|---|---|---|---|---|---|---|---|---|
| Peak flow attenuation | X | X | |||||||
| Infiltration | X | X | |||||||
| Dispersion | X | X | X | ||||||
| Evapotranspiration | X | ||||||||
| Runoff collection and usage | X | ||||||||
| Sedimentation | X | X | X | X | X | X | |||
| Flotation | X | X | X | ||||||
| Laminar separation | X | ||||||||
| Swirl concentration | X | ||||||||
| Sorption | X | ||||||||
| Filtration | X | X | X | ||||||
| Plant metabolism | X | X | X | ||||||
| Temperature reduction | X | X | X |
Unit processes of stormwater treatment techniques (Adapted from WEF, 2008)
Link to this table
| Control | Infiltration basin | Infiltration trench | Bioinfiltration | Permeable pavement | Tree box/tree trench | Enhanced turf |
|---|---|---|---|---|---|---|
| Peak flow attenuation | X | X | X | X | ||
| Runoff volume reduction | X | X | X | X | X | |
| Infiltration | X | X | X | X | X | X |
| Dispersion | ||||||
| Evapotranspiration | X | X | ||||
| Runoff collection and usage | X1 | X1 | ||||
| Sedimentation | X | X | X | |||
| Flotation | X | X | ||||
| Laminar separation | ||||||
| Swirl concentration | ||||||
| Sorption | X | X | X | X | ||
| Precipitation | X | X | X | X | ||
| Coagulation | X | X | X | X | ||
| Filtration | X | |||||
| Plant metabolism | X | X | X | |||
| Nitrification/denitrification | X | X | X | |||
| Organic compound degradation | X | X | X | X | ||
| Pathogen die off | X | X | X | |||
| Temperature reduction | X | X | X | X | ||
| Disinfection | X | X | X | X |
1 If underdrain is present
Volume control processes
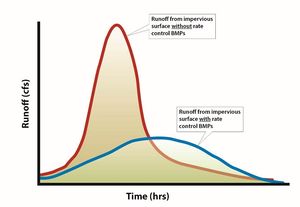
- Peak flow attenuation. Peak flow attenuation is the process by which the peak stormwater runoff discharge is reduced and the total volume of stormwater flow is spread out over a longer period of time. The result is a broad, more flat hydrograph (DNR).
- Runoff volume reduction. Runoff volume reduction means that a portion of the runoff is removed from the system, and the overall runoff volume is decreased (WEF, 2012).
- Runoff Collection. Runoff collection is the process by which runoff is captured and stored for a later use. Storage varies from cisterns and rain barrels up to large water tanks and ponds (WEF, 2012).
- Infiltration. Infiltration is the process by which the stormwater enters into the subsurface. It is different from filtration in the fact that the water is not ultimately collected by any underdrain system, but rather the infiltrated water continues to move down into the subsurface and groundwater table, or moves laterally as subsurface flow (Erickson et al., 2013). Infiltration does not only achieve volume control and peak flow attenuation, but also contributes to pollution reduction.
- Evaporation. Evaporation is the process by which water changes from a liquid to a gas. It can occur from plant interception, depression storage, and from within the soil. Evaporation from interception occurs when rain falling on a vegetative surface is intercepted by leaves or blades. The water captured then evaporates back into the atmosphere. Evaporation from depression storage occurs when the runoff is captured in puddles or ponded areas. The water trapped in these areas evaporates back into the atmosphere. Soil evaporation is different than depression storage evaporation because it occurs within the soil rather than on the surface of the soil. As water right near the surface evaporates, water from deeper in the soil profile moves upward to replace that water (WEF, 2012).
Physical processes
- Sedimentation. Sedimentation is the process by which solids are removed from the stormwater runoff via settling (Erickson et al., 2013).
- Screening. Screening is the process by which gross pollutants are removed through straining devices with large openings (WEF, 2012).
- Transpiration. Transpiration is the process by which water vapor passes into the atmosphere through the vascular system of plants (WEF, 2012).
- Filtration. Filtration is the retention of suspended particles as the water passes through a granular media or vegetation. The predominate mechanism of filtration is straining, which occurs when particles are too large to pass through the void space in the media (Erickson et al., 2012).
- Flotation. Flotation is when materials (e.g., plastic bags, petroleum hydrocarbons, light solids) remain on the surface of the water because they have a specific gravity that is less than the water (WEF, 2012).
- Temperature reduction. Temperature reduction is the process by which stormwater with a high temperature will cool due to the fact that it is in contact with a surface (i.e., ground, air, etc.) at a lower temperature (Erickson et al., 2013).
- Laminar separation. Laminar separation is a process by which certain pollutants are removed from stormwater using laminar flow conditions. Laminar conditions can be achieved by using plates or tubes to calm the water. Turbulence (even mild turbulence) will lead to a reduction in the removal of oil droplets and fine sediments from the runoff WEF, 2012).
- Swirl concentration. Swirl concentration is a process by which stormwater runoff is routed through cylindrical shaped devices that create a vortex. This swirling motion helps to enhance the separation of particles from the stormwater (WEF, 2012).
Biological processes
- Pathogen die-off. Pathogen die-off is the process by which pathogens are inactivated via death.
- Plant metabolism. Plant metabolism is the process by which plants take in certain nutrients (which can be pollutants in stormwater) to aid in the plant growth (WEF, 2012). This is one of the main mechanisms by which phosphorous is removed from stormwater runoff.
- Degradation. Degradation is the process by which pollutants are broken down into less harmful components. Bacteria often play an important role in degradation and have been found to degrade pesticides, petroleum compounds, and other anthropogenic organics.
- Nitrification/denitrification. Nitrification/denitrification is the process by which nitrogen is removed from stormwater. With nitrification, ammonium (NH4+) or ammonia (NH3) is oxidized to form nitrite (NO2-) and then nitrate (NO3-). It is an aerobic process (requires oxygen) and requires the presence of Nitrosomonas and Nitrobacters type bacteria to form nitrite and nitrate, respectively. Denitrification is the process by which nitrate is reduced to form molecular nitrogen (N2). It occurs in anaerobic conditions (no oxygen) with the aid of different types of bacteria (WEF, 2012).
Chemical processes
- Precipitation. Precipitation is the process by which inorganic dissolved species join together to form a settleable or filterable particulate. This process can occur naturally or with the aid of chemicals. Natural precipitation occurs without the purposeful addition of chemicals. For example, if the stormwater has sufficient calcium and alkalinity, calcium phosphates may form which are more easily removed from the system. Chemical precipitation is the result of the addition of a substance that will aid in the formation of the precipitates. For example, the addition of alum and sodium aluminate into water can aid in the removal of phosphorous (WEF, 2012).
- Coagulation. Coagulation is the process by which smaller particles collect together to form larger particles. This allows the particles to then settle out. Chemicals can be added to aid the removal of silts, clays, or other dissolved pollutants. The process of coagulation is twofold. First, the particles must be destabilized. Suspended small particles tend have a negative charge and repel each other and, by destabilizing the charge, the smaller particles will begin to join together. The joining of these particles is the second step, flocculation. Flocculation can occur naturally (e.g., wind in a wet basin) or mechanically (e.g. paddles) (WEF, 2012).
- Sorption. There are three types of sorption; adsorption, absorption, and ion exchange. Adsorption is when pollutants bind to the surface of a soil particle. If the chemical equilibrium changes in the future, the pollutants can desorb and continue to travel downward towards the groundwater table. With absorption, a pollutant penetrates the soil and attaches at a molecular level. With ion exchanged, existing less “interesting” ions are replaced with ions of greater interest to the media (WEF, 2012).